Abstract
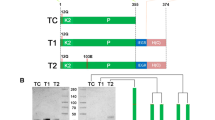
Microplasminogen (μPlg), a truncated form of human plasminogen, has considerable potential as a direct-acting thrombolytic agent. To further develop μPlg into a thrombolytic agent with anti-thrombus properties, we constructed two μPlg variants containing tripeptide Arg-Gly-Asp (RGD) and tetrapeptide Gly-Pro-Arg-Pro (GPRP) by site-directed mutagenesis. The recombinant cDNAs were expressed in yeast (Pichia pastoris) and purified to high homogeneity by Ni–NTA affinity chromatography. The specific activities of RGD-μPlg and GPRP-μPlg were 7.7 and 13.3 U/mg, respectively, as determined using the fibrin-plate method. RGD-μPlg significantly inhibited ADP-induced platelet aggregation, which was 33.6- and 14.1-fold higher than the native μPlg and GPRP-μPlg, respectively. On the other hand, GPRP-μPlg prolonged thrombin-initialized fibrinogen polymerization in a concentration-dependent manner, which was 9.2- and 5.7-fold stronger than μPlg and RGD-μPlg, respectively. Under activation by urokinase, μPlg, RGD-μPlg, and GPRP-μPlg all showed over 80 % conversions to their active enzyme in 24 h. The structure models that docked RGD-μPlg and μPlg activation loops into the enzymatic active site of urokinase showed that Pro559 to Asp559 mutation of RGD-μPlg led to an alteration in the interaction, which possibly explains the slowed activation of RGD-μPlg by urokinase over an 80-min period. In conclusion, this study has presented two recombinant μPlg variants with anti-platelet aggregation and anti-fibrinogen clotting activity, thus suggesting the anti-thrombosis properties of these two μPlg derivatives.







Similar content being viewed by others
Abbreviations
- UK:
-
Urokinase
- Plm:
-
Plasmin
- t-PA:
-
Tissue-type plasminogen activator
- Plg:
-
Plasminogen
- μPlg:
-
Microplasminogen
References
Verheugt FW, Meijer A, Lagrand WK, Van Eenige MJ (1996) Reocclusion: the flip side of coronary thrombolysis. J Am Coll Cardiol 27:766–773
Laudano AP, Doolittle RF (1978) Synthetic peptide derivatives that bind to fibrinogen and prevent the polymerization of fibrin monomers. Proc Natl Acad Sci USA 75:3085–3089
Stabenfeldt SE, Aboujamous NM, Soon AS, Barker TH (2011) A new direction for anticoagulants: inhibiting fibrin assembly with PEGylated fibrin knob mimics. Biotechnol Bioeng 108:2424–2433
Plow EF, Pierschbacher MD, Ruoslahti E, Marguerie GA, Ginsberg MH (1985) The effect of Arg-Gly-Asp-containing peptides on fibrinogen and von Willebrand factor binding to platelets. Proc Natl Acad Sci USA 82:8057–8061
Bi Q, Cen X, Huang Y, Zhu S (2002) Construction and characterization of trifunctional single-chain urokinase-type plasminogen activators. Eur J Biochem 269:1708–1713
Bingxing S, Aiping Y, Yuying L, Li J, Jin J, Dong C, Wu C (2007) Locally activity-released bifunctional fusion protein enhances antithrombosis and alleviates bleeding risk. J Thromb Thrombolysis 24:283–292
Anmol K, Krishna Kanth P, Candasamy M, Kotra S, Rao KR (2013) Evaluation of a multifunctional staphylokinase variant with thrombin inhibition and antiplatelet aggregation activities produced from salt-inducible E. coli GJ1158. Can J Physiol Pharmacol 91:839–847
Novokhatny VV, Jesmok GJ, Landskroner KA, Marder VJ, Zimmerman TP (2004) Locally delivered plasmin: why should it be superior to plasminogen activators for direct thrombolysis. Trends Pharmacol Sci 25:72–75
Daphne S, Mansze K, Valery N, Jesmok G, Marder VJ (2003) Distinct dose-dependent effects of plasmin and TPA on coagulation and hemorrhage. Blood 101:3002–3007
Marder V (2008) Pre-clinical studies of plasmin: superior benefit-to-risk ratio of plasmin compared to tissue plasminogen activator. Thromb Res 1223:S9–S15
Thijs VN, Peeters A, Vosko M, Aichner F, Schellinger PD, Schneider D, Neumann-Haefelin T, Röther J, Davalos A, Wahlgren N, Verhamme P (2009) Randomized, placebo-controlled, dose-ranging clinical trial of intravenous microplasmin in patients with acute ischemic stroke. Stroke 40:3789–3795
Marder VJ, Manyak S, Gruber T, Goyal A, Moreno G, Hunt J, Bromirski J, Scuderi P, Petteway SR Jr, Novokhatny V (2010) Haemostatic safety of a unique recombinant plasmin molecule lacking kringles 2-5. J Thromb Haemost 104:780–787
Peter V, Martine J, Godelieve G, Devis J, Maleux G, Stas M (2009) A pilot trial of microplasmin in patients with long-term venous access catheter thrombosis. J Thromb Haemost 28:477–481
Fu J, Ren J, Zou L, Bian G, Li R, Lu Q (2008) The thrombolytic effect of miniplasmin in a canine model of femoral artery thrombosis. Thromb Res 122:683–690
Varma R, Haller JA, Kaiser PK (2015) Improvement in patient-reported visual function after ocriplasmin for vitreomacular adhesion: results of the microplasmin for intravitreous injection–traction release without surgical treatment (mivi-trust) trials. JAMA Ophthalmol 133:97–100
Shi GY, Wu HL (1988) Isolation and characterization of microplasminogen. A low molecular weight form of plasminogen. J Biol Chem 263:17071–17075
Wu HL, Shi GY, Bender ML (1988) Preparation and purification of microplasmin. Proc Natl Acad Sci USA 84(23):8292–8295
Liu R, Bing Z, Zhang Y, Gu J, Yu M, Song H, Yu M, Mo W (2015) High-level expression, purification, and enzymatic characterization of truncated human plasminogen (Lys531-Asn791) in the methylotrophic yeast Pichia pastoris. BMC Biotechnol 15:50. doi:10.1186/s12896-015-0179-z
Astrup T, Mullertz S (1952) The fibrin plate method for estimating fibrinolytic activity. Arch Biochem Biophys 40:346–351
Walkowiak B, Kralisz U, Michalec L, Majewska E, Koziolkiewicz W, Ligocka A, Cierniewski CS (2000) Comparison of platelet aggregability and P-selectin surface expression on platelets isolated by different methods. Thromb Res 99:495–502
Wang X, Terzyan S, Tang J, Loy JA, Lin X, Zhang XC (2000) Human plasminogen catalytic domain undergoes an unusual conformational change upon activation. J Mol Biol 295:903–914
Spraggon G, Phillips C, Nowak UK, Ponting CP, Saunders D, Dobson CM, Stuart DI, Jones EY (1995) The crystal structure of the catalytic domain of human urokinase-type plasminogen activator. Structure 3:681–691
Ewald GA, Eisenberg PR (1995) Plasmin-mediated activation of contact system in response to pharmacological thrombolysis. Circulation 91:28–36
Chang WC, Shi GY, Chow YH, Chang LC, Hau JS, Lin MT, Jen CJ, Wing LY, Wu HL (1993) Human plasmin induces a receptor- mediated arachidonate release coupled with G proteins in endothelial cells. Am J Physiol 264:C271–C281
Kimura M, Andersen TT, Fenton JW 2nd, Bahou WF, Aviv A (1996) Plasmin-platelet interaction involves cleavage of functional thrombin receptor. Am J Physiol 271:C54–C60
Ogiwara K, Nogami KK, Shima M (2010) Plasmin-induced procoagulant effects in the blood coagulation: a crucial role of coagulation factors V and VIII. Blood Coagul Fibrinolysis 21:568–576
Christensen U, Bangert K, Thorsen S (1996) Reaction of human alpha2- antiplasmin and plasmin stopped-flow fluorescence kinetics. FEBS Lett 387:58–62
Nagai N, Demarsin E, Van Hoef B, Wouters S, Cingolani D, Laroche Y, Collen D (2003) Recombinant human microplasmin: production and potential therapeutic properties. J Thromb Haemost 1:307–313
Suzuki Y, Nagai N, Collen D (2004) Comparative effects of microplasmin and tissue-type plasminogen activator (tPA) on cerebral hemorrhage in a middle cerebral artery occlusion model in mice. J Thromb Haemost 2:1617–1621
Acknowledgments
This work was supported by Grants B2013105 from Chinese Hubei Provincial Department of Education and grants 81102502 from Chinese National Natural Science Funds. We are grateful to Shuang Zhu for providing the electroporator and Yun Xiao for anti-platelet aggregation analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing financial interest.
Rights and permissions
About this article
Cite this article
Chen, W., Li, Y., Chen, P. et al. In vitro fibrinolysis and antithrombosis characterizations of novel recombinant microplasminogen with RGD and GPRP peptides. J Thromb Thrombolysis 42, 118–126 (2016). https://doi.org/10.1007/s11239-016-1334-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11239-016-1334-7