Abstract
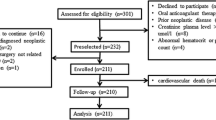
Abnormal platelet reactivity is associated with recurrent ischemia and bleeding following percutaneous coronary intervention (PCI). Protease-activated receptor-1 (PAR1), encoded by F2R, is a high affinity thrombin receptor on platelets and the target of the antiplatelet drug vorapaxar. The intronic single nucleotide polymorphism F2R IVS-14 A/T affects PAR1 receptor density and function. We hypothesized that carriers of the T allele, who have been shown to have decreased platelet reactivity, would be at lower risk for thrombotic events, but higher risk for bleeding following PCI. Using BioVU, the Vanderbilt DNA repository linked to the electronic medical record, we studied 660 patients who underwent PCI for unstable or stable coronary artery disease. Primary outcome measures were major adverse cardiovascular events (MACE, composite of revascularization, MI, stroke, death) and bleeding (assessed by Bleeding Academic Research Consortium scale) over 24 months. The minor allele (T) frequency was 14.8 %. There were no genotypic differences in the frequency of MACE (33.7, 28.8, and 31.6 % for A/A, A/T, and T/T respectively, P = 0.50) or bleeding (15.7, 14.7, and 18.8 % for A/A, A/T, and T/T respectively, P = 0.90). In a Cox regression model, fully adjusted for age, race, sex, BMI, and smoking status, carrying a T allele was not associated with MACE (HR 1.19, 95 % CI 0.89–1.59, P = 0.23) or bleeding (HR 0.73, 95 % CI 0.37–1.4, P = 0.34). In conclusion, in our population, F2R IVS-14 PAR1 variability does not affect risk of MACE or bleeding following PCI.



Similar content being viewed by others
References
Tantry US, Bonello L, Aradi D et al (2013) Consensus and update on the definition of on-treatment platelet reactivity to adenosine diphosphate associated with ischemia and bleeding. J Am Coll Cardiol 62:2261–2273
Trenk D, Hochholzer W, Fromm MF et al (2008) Cytochrome P450 2C19 681G > A polymorphism and high on-clopidogrel platelet reactivity associated with adverse 1-year clinical outcome of elective percutaneous coronary intervention with drug-eluting or bare-metal stents. J Am Coll Cardiol 51:1925–1934
Harmsze A, van Werkum JW, Bouman HJ et al (2010) Besides CYP2C19*2, the variant allele CYP2C9*3 is associated with higher on-clopidogrel platelet reactivity in patients on dual antiplatelet therapy undergoing elective coronary stent implantation. Pharmacogenet Genomics 20:18–25
Simon T, Verstuyft C, Mary-Krause M et al (2009) Genetic determinants of response to clopidogrel and cardiovascular events. N Engl J Med 360:363–375
Collet JP, Cuisset T, Range G et al (2012) Bedside monitoring to adjust antiplatelet therapy for coronary stenting. N Engl J Med 367:2100–2109
Bray PF (2007) Platelet hyperreactivity: predictive and intrinsic properties. Hematol Oncol Clin North Am 21:633–645, v–vi
Coughlin SR (2005) Protease-activated receptors in hemostasis, thrombosis and vascular biology. J Thromb Haemost 3:1800–1814
Leger AJ, Covic L, Kuliopulos A (2006) Protease-activated receptors in cardiovascular diseases. Circulation 114:1070–1077
Lasne D, Krenn M, Pingault V et al (1997) Interdonor variability of platelet response to thrombin receptor activation: influence of PlA2 polymorphism. Br J Haematol 99:801–807
Dupont A, Fontana P, Bachelot-Loza C et al (2003) An intronic polymorphism in the PAR-1 gene is associated with platelet receptor density and the response to SFLLRN. Blood 101:1833–1840
Smith SM, Judge HM, Peters G et al (2005) PAR-1 genotype influences platelet aggregation and procoagulant responses in patients with coronary artery disease prior to and during clopidogrel therapy. Platelets 16:340–345
Sibbing D, Schulz S, Braun S et al (2010) Antiplatelet effects of clopidogrel and bleeding in patients undergoing coronary stent placement. J Thromb Haemost 8:250–256
Cuisset T, Cayla G, Frere C et al (2009) Predictive value of post-treatment platelet reactivity for occurrence of post-discharge bleeding after non-ST elevation acute coronary syndrome. Shifting from antiplatelet resistance to bleeding risk assessment? EuroIntervention 5:325–329
Campo G, Parrinello G, Ferraresi P et al (2011) Prospective evaluation of on-clopidogrel platelet reactivity over time in patients treated with percutaneous coronary intervention relationship with gene polymorphisms and clinical outcome. J Am Coll Cardiol 57:2474–2483
Parodi G, Bellandi B, Venditti F et al (2012) Residual platelet reactivity, bleedings, and adherence to treatment in patients having coronary stent implantation treated with prasugrel. Am J Cardiol 109:214–218
Delaney JT, Ramirez AH, Bowton E et al (2012) Predicting clopidogrel response using DNA samples linked to an electronic health record. Clin Pharmacol Ther 91:257–263
Cunningham A, Stein CM, Chung CP, Daugherty JR, Smalley WE, Ray WA (2011) An automated database case definition for serious bleeding related to oral anticoagulant use. Pharmacoepidemiol Drug Saf 20:560–566
Mehran R, Rao SV, Bhatt DL et al (2011) Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the Bleeding Academic Research Consortium. Circulation 123:2736–2747
Rao SV, Dai D, Subherwal S et al (2012) Association between periprocedural bleeding and long-term outcomes following percutaneous coronary intervention in older patients. JACC Cardiovasc Interv 5:958–965
Ndrepepa G, Schuster T, Hadamitzky M et al (2012) Validation of the Bleeding Academic Research Consortium definition of bleeding in patients with coronary artery disease undergoing percutaneous coronary intervention. Circulation 125:1424–1431
Hulot JS, Bura A, Villard E et al (2006) Cytochrome P450 2C19 loss-of-function polymorphism is a major determinant of clopidogrel responsiveness in healthy subjects. Blood 108:2244–2247
Mega JL, Close SL, Wiviott SD et al (2009) Cytochrome p-450 polymorphisms and response to clopidogrel. N Engl J Med 360:354–362
Shuldiner AR, O’Connell JR, Bliden KP et al (2009) Association of cytochrome P450 2C19 genotype with the antiplatelet effect and clinical efficacy of clopidogrel therapy. JAMA 302:849–857
Stone GW, Witzenbichler B, Weisz G et al (2013) Platelet reactivity and clinical outcomes after coronary artery implantation of drug-eluting stents (ADAPT-DES): a prospective multicentre registry study. Lancet 382:614–623
Frelinger AL 3rd, Bhatt DL, Lee RD et al (2013) Clopidogrel pharmacokinetics and pharmacodynamics vary widely despite exclusion or control of polymorphisms (CYP2C19, ABCB1, PON1), noncompliance, diet, smoking, co-medications (including proton pump inhibitors), and pre-existent variability in platelet function. J Am Coll Cardiol 61:872–879
Price MJ, Carfson AR, Murray SS et al (2012) First pharmacogenomic analysis using whole exome sequencing to identify novel genetic determinants of clopidogrel response variability: results of the Genotype Information and Functional Testing (GIFT) Exome Study. J Am Coll Cardiol 59:E9
Bhatt DL (2009) Prasugrel in clinical practice. N Engl J Med 361:940–942
Menown IB (2011) Aspirin, P2Y12 blockers, cilostazol, PAR-1 blockers and emerging antiplatelet therapies: can biomarkers guide clinical development and practice? Biomark Med 5:1–3
Chhatriwalla AK, Amin AP, Kennedy KF et al (2013) Association between bleeding events and in-hospital mortality after percutaneous coronary intervention. JAMA 309:1022–1029
Price MJ, Berger PB, Teirstein PS et al (2011) Standard- vs high-dose clopidogrel based on platelet function testing after percutaneous coronary intervention: the GRAVITAS randomized trial. JAMA 305:1097–1105
Storey RF, Kotha J, Smyth SS et al (2014) Effects of vorapaxar on platelet reactivity and biomarker expression in non-ST-elevation acute coronary syndromes. The TRACER Pharmacodynamic Substudy. Thromb Haemost 111:883–891
Cleator JH, Duvernay MT, Holinstat M et al (2014) Racial differences in resistance to P2Y12 receptor antagonists in type 2 diabetic subjects. J Pharmacol Exp Ther 351:33–43
Behan MW, Fox SC, Heptinstall S, Storey RF (2005) Inhibitory effects of P2Y12 receptor antagonists on TRAP-induced platelet aggregation, procoagulant activity, microparticle formation and intracellular calcium responses in patients with acute coronary syndromes. Platelets 16:73–80
Tricoci P, Huang Z, Held C et al (2012) Thrombin-receptor antagonist vorapaxar in acute coronary syndromes. N Engl J Med 366:20–33
Morrow DA, Braunwald E, Bonaca MP et al (2012) Vorapaxar in the secondary prevention of atherothrombotic events. N Engl J Med 366:1404–1413
Scirica BM, Bonaca MP, Braunwald E et al (2012) Vorapaxar for secondary prevention of thrombotic events for patients with previous myocardial infarction: a prespecified subgroup analysis of the TRA 2 degrees P-TIMI 50 trial. Lancet 380:1317–1324
Cavender MA, Scirica BM, Bonaca MP et al (2015) Vorapaxar in patients with diabetes mellitus and previous myocardial infarction: findings from the thrombin receptor antagonist in secondary prevention of atherothrombotic ischemic events-TIMI 50 trial. Circulation 131:1047–1053
Bonaca MP, Scirica BM, Braunwald E et al (2014) Coronary stent thrombosis with vorapaxar versus placebo: results from the TRA 2 degrees P-TIMI 50 trial. J Am Coll Cardiol 64:2309–2317
Kawai VK, Cunningham A, Vear SI et al (2014) Genotype and risk of major bleeding during warfarin treatment. Pharmacogenomics 15:1973–1983
Author information
Authors and Affiliations
Corresponding author
Additional information
Work supported by Vanderbilt Institute for Clinical and Translational Research Award to JHC.
Rights and permissions
About this article
Cite this article
Friedman, E.A., Texeira, L., Delaney, J. et al. Evaluation of the F2R IVS-14A/T PAR1 polymorphism with subsequent cardiovascular events and bleeding in patients who have undergone percutaneous coronary intervention. J Thromb Thrombolysis 41, 656–662 (2016). https://doi.org/10.1007/s11239-015-1285-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11239-015-1285-4