Abstract
Background
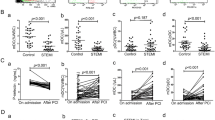
Some factors play pathogenic roles in the development of restenosis after percutaneous coronary intervention (PCI). We measured and compared the ratio of elevated levels of monocytic chemotactic peptide-1 (MCP-1), regulated on activation normally T-cell expressed and secreted (RANTES), soluble (s) P-selectin, sE-selectin and adiponectin after PCI.
Methods
Plasma levels of chemokines and soluble markers were measured before and 30 days after PCI in 96 patients (69 males and 27 females, aged 63 ± 9 years) who underwent PCI and who had repeated angiograms at a 6-month follow-up. In addition, we carried out the basic study of the tissue factor expression on monocytic cell line (THP-1) by MCP-1.
Results
Restenosis occurred in 33 (34.4%) patients. A significant and time-dependent increase in MCP-1 was observed in the restenosis group. However, there were no significant differences in RANTES, sP-selectin, and sE-selectin levels with or without restenosis. Adiponectin levels in patients with coronary artery disease were significantly lower than levels in normal controls. However, adiponectin levels were no different at baseline between patients with or without restenosis. MCP-1 did not induce the expression of tissue factor on THP-1. However, the recombinant sCD40 ligand-induced expression of tissue factor on THP-1 was enhanced by the addition of MCP-1.
Conclusion
These findings suggest that restenosis development after PCI in patients with coronary artery disease may involve the participation of MCP-1 after PCI, and adiponectin incompletely prevent this MCP-1-dependent restenosis.




Similar content being viewed by others
References
Smith SC, Dove JT, Jacobs AK et al (2001) ACC/AHA Guidelines for percutaneous coronary intervention (revision of the 1993 PTCA guidelines)––executive summary. Circulation 103:3019–3041
Califf RN, Fortin DF, Frid DJ et al (1991) Restenosis after coronary angioplasty: an overview. J Am Coll Cardiol 17:2B–13B
Fitzgerald DJ, Roy I, Catella F, FitzGerald GA (1986) Platelet activation in unstable angina. N Engl J Med 315:983–989
Liu M, Roubin G, King S (1989) Restenosis after coronary angioplasty: Potential biologic determinants and role of intimal hyperplasia. Circulation 79:1374–1387
Ross R (1999) Atherosclerosis––an inflammatory disease. N Engl J Med 340:115–126
Nelken N, Coughlin S, Gordon D, Wilcox JN (1991) Monocyte chemoattractant protein-1 in human atheromatous plaques. J Clin Invest 88:1121–1127
Yla-Herttuala S, Lipton B, Rosenfeld M et al (1991) Expression of monocyte chemoattractant protein-1 in macrophage-rish areas of human and rabbit atherosclerotic lesions. Proc Natl Acad Sci 80:5252–5256
Reckless J, Rubin E, Verstuyft J, Metcalfe JC, Grainger DJ (1999) Monocyte chemoattractant protein-1, but not tumor necrosis factor is correlated with monocyte infiltation in mouse lipid lesions. Circulation 99:2310–2316
Cipollone F, Marini M, Fazia M et al (2001) Elevated circulating levels of monocyte chemoattractant protein-1 in patients with restenosis after coronary angioplasty. Arterioacler Thromb Vasc Biol 21:327–334
Maeda K, Okubo K, Shimomura I et al (1996) cDNA cloning and expression of a novel adipose specific collgen-like factor, apM1 (AdiPose Most abundant Gene transcript 1). Biochem Biophys Res Commun 221:286–289
Ouchi N, Kihara S, Arita Y et al (1999) Novel modulator for endothelial adhesion molecules; adipocyte-derived plasma protein adiponectin. Circulation 100:2473–2476
Ouchi N, Kihara S, Arita Y et al (2000) Adiponectin, an adipocyte-derived plasma protein, inhibits endothelial NF-B signaling through a camp-dependent pathway. Circulation 102:1296–1301
Ouchi N, Kihara S, Arita Y et al (2001) Adipocyte-derived plasma protein, adiponectin, suppresses lipid accumulation and class A scavenger receptor expression in human monocyte-derived macropages. Circulation 103:1057–1063
Okamoto Y, Arita Y, Nishida M et al (2000) An adipocyte-derived plasma protein, adiponectin, adheres to injured vascular walls. Horm Metab Res 32:47–50
Arita Y, Kihara S, Ouchi N et al (2002) Adipocyte-derived plasma protein adiponectin acts a platelet-derived growth factor-BB-binding protein and regulates growth factor-induced common postreceptor signal in vascular smooth muscle cell. Circulation 105:2893–2898
Rollins BJ (1996) Monocyte chemoattractant protein-1: a potent regulator of monocyte recruitment in inflammatory disease. Mol Med Today 2:198–204
Carr MW, Roth SJ, Luther E, Rose SS, Springer TA (1994) Monocyte chemoattractant protein-1 acts as a T-lymphocyte chemoattractant. Proc Natl Acad Sci 91:3652–3656
Aukrust P, Ueland T, Muller F et al (1998) Elevated circulating levels of C-C chemokines in patients with congestive heart failure. Circulation 97:1136–1143
Conti P, Pang X, Boucher W, Letourneau R, Reale M (1997) Impact of RANTES and MCP-1 chemokines on in vivo mast cell recruitment in rat skin injection model and their role in modifying the protein and mRNA levels for histidine decarboxylase. Blood 89:4120–4127
Ueda A, Okuda K, Ohno S et al (1994) NF-kB and Splregulate transcription of the human monocyte chemoattractant protein-1 gene. J Immunol 153:2052–2063
Taubman MB, Rollins BJ, Poon M et al (1992) mRNA accumulates rapidly in aortic injury and in platelet-derived growth factor-stimulated vascular smooth muscle cells. Cir Res 70:314–325
Martinovic I, Abegunewardene N, Seul M et al (2005) Elevated monocyte chemoattractant protein-1 serum levels in patients at risk for coronary artery disease. Cir J 69:1484–1489
Arita Y, Kihara S, Ouchi N et al (1999) Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun 257:79–83
Hotta K, Funahashi T, Arita Y et al (2000) Plasma concentrations of a novel adipo-specific protein, adiponectin, in type 2 diabetic patients. Arterioscler Thromb Vasc Biol 20:1595–1599
Matsuda M, Shimomura I, Sata M et al (2002) Role of adiponectin in preventing vascular stenosis—the missing link of adipo-vascular axis. J Biol Chem 277:37487–37491
Kojima S, Funahashi T, Sakamoto T et al (2003) The variation of plasma concentrations of a novel, adipocyte derived protein, adiponectin, in patients with acute myocardial infarction. Heart 89:667
Nakamura Y, Shimada K, Fukuda D et al (2004) Implications of plasma concentrations of adiponectin in patients with coronary artery disease. Heart 90:528–533
Stejskal D, Bartek J (2003) Adiponectin in patients with various stages of coronary heart disease––comparison of its concentration in coronary arteries and peripheral venous circulation. Biomed Pap Med Fuc Univ Palacky Ulomouc Czech Repub 147:161–166
Olijhoek JK, Koerselman J, de Jaegere KPPTh et al (2005) Presence of the metabolic syndrome does not impair coronary collateral vessel formation in patients with documented coronary artery disease. Diabetes Care 28:683–689
Charo IF, Taubman MB (2004) Chemokines in the pathogenesis of vascular disease. Circ Res 95:858–866
Sakamoto T, Ishibashi T, Sakamoto N et al (2005) Endogeneous NO blockade enhances tissue factor expression via increased Ca2+ influx through MCP-1 in endothelial cells by monocyte adhesion. Artherioscler Thromb Vasc Biol 25:2005–2011
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Inami, N., Nomura, S., Shimazu, T. et al. Adiponectin incompletely prevent MCP-1-dependent restenosis after percutaneous coronary intervension in patients with coronary artery disease. J Thromb Thrombolysis 24, 267–273 (2007). https://doi.org/10.1007/s11239-007-0042-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11239-007-0042-8