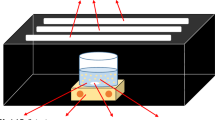
Samples of nanocrystalline graphite-like carbon nitride (CGCN) modified with magnesium chloride have been obtained. The synthesized Mg-CGCN samples have been characterized by UV and IR spectroscopies, X-ray diffraction, energy dispersive X-ray analysis, and scanning electron microscopy. It is found that Mg-CGCN exhibits 60% higher photocatalytic activity in the reaction of hydrogen evolution from aqueous ethanol solutions under visible light than undoped CGCN. This may be related to a change in electrophysical characteristics (potentials of allowed bands) resulting from the incorporation of Mg2+ ions into the CGCN structure, improved dissociation of photogenerated charges, and inhibition of their recombination.


Similar content being viewed by others
References
X. Wang, K. Maeda, A. Thomas, et al., Nat. Mater., 8, No. 1, 76-80 (2009), https://doi.org/10.1038/nmat2317.
X. Wang, K. Maeda, X. Chen, et al., J. Am. Chem Soc.,131, No. 5, 1680-1681 (2009), https://doi.org/10.1021/ja809307s.
K. Maeda, X. Wang,Y. Nishihara, et al., J. Phys. Chem. C.,113, No. 12, 4940-4947 (2009), https://doi.org/10.1021/jp809119m.
X. Chen, Y. S. Jun, K. Takanabe, et al., Chem. Mater.,21, No. 18, 4093-4095 (2009), https://doi.org/10.1021/cm902130z.
J. Wen, J. Xie, X. Chen, and X. Li, Appl. Surf. Sci. B., 391, 72-123 (2017), https://doi.org/10.1016/j.apsusc.2016.07.030.
O. L. Stroyuk, A. E. Raevskaya, and S. Ya. Kuchmy, Theor. Exp. Chem., 54, No. 1, 1-35 (2018), https://doi.org/10.1007/s11237-018-9541-2.
O. L. Stroyuk, A. E. Raevskaya, and S. Ya. Kuchmy, Theor. Exp. Chem., 55, No. 3, 147-172 (2019), https://doi.org/10.1007/s11237-019-09607-4.
J. Wang, P. Kumar, and H. Zhao, Green Chem., 23, No. 19, 7435-7457 (2021).
G. Grodziuk, N. Shcherban, V. Shvalagin, et al., Int. J. Hydrog. Energy, 42, No. 38, 24108-24116 (2017), https://doi.org/10.1016/j.ijhydene.2017.07.238.
G. Ya. Grodzyuk, V. V. Shvalagin, N. S. Andryushina, et al., Theor. Exp. Chem., 54, No. 2, 99-106 (2018), https://doi.org/10.1007/s11237-018-9552-z.
L. Lin, H. Ou, Y. Zhang, and X. Wang, ACS Catal., 6, No. 6, 3921-3931 (2016), https://doi.org/10.1021/acscatal.6b00922.
H. Ou, L. Lin, Y. Zheng, et al., Adv. Mater., 29, No. 22, 1700008 (2017), .
L. Lin, W. Ren, C. Wang, et al., Appl. Catal. B., 231, 234-241 (2018), 10.10167j.apcatb.2018.03.009.
N. Andriushyna, V. Shvalagin, A. Korzhak, et al., Appl. Surf. Sci., 475, 348-354 (2019), https://doi.org/10.1016/j.apsusc.2018.12.287.
V. V. Shvalagin, G. V. Korzhak, S. Ya. Kuchmiy, et al., J. Photochem. Photobiol. A., 390, 112295 (2020), https://doi.org/10.1016/j.jphotochem.2019.112295.
J.-Y. Tang, W.-G. Zhou, R.-T. Guo, et al., Catal. Commun., 107, 92-95 (2018), 10.10167j.catcom.2018.01.006.
S. B. Vuggili, S. K. Khanth, K. Kadiya, et al., J. Environ. Chem. Eng., 7, No. 6, 103440 (2019), https://doi.org/10.1016/j.jece.2019.103440.
X. Dong, S. Zhang, H. Wu, et al., RSC Adv., 9, No. 49, 28894-28901 (2019), https://doi.org/10.1039/C9RA04606B.
W. Yan, L. Li Yan, C. Jing, et al., Appl. Catal. B., 244, 475-485 (2019), https://doi.org/10.1016/j.apcatb.2018.11.069.
D. Long, W. Diao, X. Rao, and Y. Zhang, ACS Appl. Energy Mater., 3, No. 9, 9278-9284 (2020), https://doi.org/10.1021/acsaem.0c01619.
S. K. Sahoo, I. F. Teixeira, A. Naik, et al., J. Phys. Chem. C.,125, No. 25, 13749-13758 (2021), https://doi.org/10.1021/acs.jpcc.1c03947.
V. Shvalagin, S. Kuchmiy, M. Skoryk, et al., Mater. Sci. Eng. B., 271, 115304 (2021), https://doi.org/10.1016/j.mseb.2021.115304.
D. C. Bradley and M. N. Gitlitz, J. Chem. Soc., 980-984 (1969), https://doi.org/10.1039/j19690000980.
J. Yu, K. Wang, W. Xiao, et al., Phys. Chem. Chem. Phys., 16, No. 23, 11462-11469 (2014), https://doi.org/10.1039/c4cp00133h.
A. Savateev, S. Pronkin, J. D. Epping, et al., ChemCatChem., 9, No. 1, 167-174 (2017), https://doi.org/10.1016/j.jssc.2013.02.016.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Teoretychna ta Eksperymentalna Khimiya, Vol. 58, No. 3, pp. 179-184, May-June, 2022.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Grodzyuk, G.Y., Koryakina, K.V., Shvalagin, V.V. et al. Photocatalytic Evolution of Hydrogen from Alcohol–Aqueous Solutions using Nanocrystalline Carbon Nitride Modified with Magnesium Chloride Under the Visible Light Irradiation. Theor Exp Chem 58, 198–204 (2022). https://doi.org/10.1007/s11237-022-09736-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11237-022-09736-3