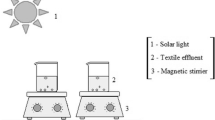
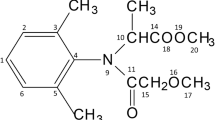
The influence of the concentration of the catalyst zinc oxide (0.01-0.3 g∙L–1) and the initial concentration of the azo dye Solophenyl Brown AGL (SB AGL, 5-75) on the efficiency of its photocatalytic degradation was studied. Almost complete degradation of the pollutant is achieved at a natural pH and catalyst content of 0.05 g∙L–1. The results of kinetic studies correspond to the Langmuir–Hinshelwood model. The efficiency of predicting the yield of photocatalytic degradation of SB AGL using a three-layer neural network with four input neurons, eight hidden neurons, and one output neuron is shown. The treatment of industrial wastewater containing two textile dye, Solophenyl Brown AGL and Cibacete Brown JNH, using a hybrid process (adsorption/solar photocatalysis) was successfully achieved.








Similar content being viewed by others
References
B. Boutra and M. Trari, Water Sci. Technol., 75, 1211-1220 (2017).
L. Penboon, A. Khrueakham, and S. Sairiam, Water Sci. Technol., 79, 958-966 (2019). https://doi.org/10.2166/wst.2019.023.
B. Boutra, M. Trari, N. Nassrallah, and B. Bellal, Theor. Exp. Chem., 52, 303-309 (2016).
N. Chekir, D. Tassalit O. Benhabiles, et al., Int. J. Hydrogen Energy (2016). https://doi.org/10.1016/j.ijhydene.2016.11.057.
S. Gita, A. Hussan, and T. G. Choudhury, Environ. Ecol., 35, No. 3C, 2349-2353 (2017).
M. R. Al-Mamun., S. Kader, M. S. Islam, and M. Z. H Khan, J. Environ. Chem. Eng., 7, 103248 (2019). https://doi.org/10.1016/j.jece.2019.103248.
S. Cheriyamundath and S. L. Vavilala, Water Environ. J., 35, 123-132 (2021). https://doi.org/10.1111/wej.12610.
S. Boumaza, F. Kaouah, D. Hamane, et al., J. Mol. Catal. A, 393, 156-165 (2014).
I. Vishan, A. Laha, and A. Kalamdhad, Water Sci. Technol., 75, 1071-1083 (2017). https://doi.org/10.2166/wst.2016.590.
H. D. Bouras, Z. Isik, E. B. Arikan, et al., Biochem. Eng. J., 146, 150-159 (2019).
L. N. Skvortsova, K. A. Bolgaru, M. V. Sherstoboeva, and K. A. Dychko, Russ. J. Phys. Chem., 94, 1248-1253 (2020). https://doi.org/10.1134/S0036024420060242.
H. Kais, N. Y. Mezenner, M. Trari, and F. Madjene, Russ. J. Phys. Chem., 93, 2834-2841 (2019). https://doi.org/10.1134/S0036024419130119.
S. Igoud, D. Zeriri, L. Aoudjit, et al., Irrig. Drain., 70, 243-253 (2021). https://doi.org/10.1002/ird.2540.
A. O. Ibhadon and P. Fitzpatrick, Catalysts, 3, 189-218 (2013).
A. Sebti, F. Souahi, F. Mohellebi, and S. Igoud, Water Sci. Technol., 76, 311-322 (2017). https://doi.org/10.2166/wst.2017.201.
B. Boutra, G. Nuray, O. Mahmut, and T. Mohamed, J. Magn. Magn. Mater., 165994, 1-11 (2020).
A. R. Khataee, M. N. Pons, and O. Zahraa, Water Sci. Technol., 62, 1112-1120 (2010). .
G. Yashni, A. Al-Gheethi, R. Mohamed, et al., Water Environ. J., 35, 190-217 (2021). https://doi.org/10.1111/wej.12619.
S. Dutta, A. Ghosh, H. Kabir, and R. Saha, Water Sci. Technol., 74, 698-713 (2016). https://doi.org/10.2166/wst.2016.248.
K. S. Ranjith and R. T. Rajendra Kumar, RSC Adv., 7, 4983-4992 (2017). https://doi.org/10.1039/C6RA27380G.
H. A. Hamad, W. A. Sadik, and M. M. Abd El-Latif, J. Environ. Sci., 43, 26-39 (2016). https://doi.org/10.1016/j.jes.2015.05.033.
F. Madjene and N. Yeddou-Mezenner, Sep. Sci. Technol. (2017). https://doi.org/10.1080/01496395.2017.1384014.
T. Bolanca, S. Ukic, I. Peternel, et al., Indian J. Chem. Technol., 21, 21-29 (2014).
J. Garcia-Alba, J. F. Barcena, C. Ugarteburu, and A. Garcia, Water Res., 150, 283-295 (2019). https://doi.org/10.1016/j.watres.2018.11.063.
D. Krishna and R. P. Sree, Indian Chem. Eng., 55, 200-222 (2013). https://doi.org/10.1080/00194506.2013.829257.
E. S. Elmolla, M. Chaudhuri. The use of artificial neural network (ANN) for modelling, simulation and prediction of advanced oxidation process performance in recalcitrant wastewater treatment, INTECH Open Access Publisher (2011).
S. M. A. Burney, T. A. Jilani, and C. Ardil, A Comparison of First and Second Order Training Algorithms for Artificial Neural Networks, International Conference on Computational Intelligence, 12-18 (2004).
A. Hassani, A. Khataee, and S. Karaca, J. Mol. Catal. A, 409, 149-161 (2015). https://doi.org/10.1016/j.molcata.2015.08.020.
J.-M. Herrmann, Top. Catal., 34, 49-65 (2005). https://doi.org/10.1007/s11244-005-3788-2.
K. M. Reza, A. Kurny, and F. Gulshan, Appl. Water Sci., 7, 1569-1578 (2017). https://doi.org/10.1007/s13201-015-0367-y.
W. A. L. Venancio, C. Rodrigues-Silva, M. G. Maniero, and J. R. Guimaraes, Water Sci. Technol., 78, 1668-1678 (2018). https://doi.org/10.2166/wst.2018.443.
A. P. Toor, A. Verma, C. K. Jotshi, et al., Dyes Pigm., 68, 53-60 (2006). https://doi.org/10.1016/j.dyepig.2004.12.009.
D. Rajamanickam and M. Shanthi, Arab. J. Chem., 9, S1858-S1868 (2016). https://doi.org/10.1016/j.arabjc.2012.05.006.
M. da Motta, R. Pereira, M. Madalena Alves, and L. Pereira, Water Sci. Technol., 70, 1670 (2014). https://doi.org/10.2166/wst.2014.428.
This work was performed at EVER’s laboratory (Epurationet Valorisation des Eaux de Rejet) and was financially supported by Unite de Developpement des Equipements Solaires, UDES/Centre de Developpement des Energies Renouvelables, CDER.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Teoretychna ta Eksperymentalna Khimiya, Vol. 57. No. 3, pp. 191-198, May-June, 2021.
Rights and permissions
About this article
Cite this article
Boutra, B., Sebti, A. & Trari, M. Photocatalytic Treatment of Synthetic and Real Textile Wastewater Using Zinc Oxide Under the Action of Sunlight. Theor Exp Chem 57, 226–236 (2021). https://doi.org/10.1007/s11237-021-09692-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11237-021-09692-4