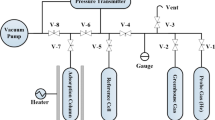
We have shown that varying the substituents in the cationic component of porous crystal compounds formed by azamacrocyclic nickel(II) complexes and the 1,3,5-benzenetricarboxylate anion has a substantial effect on their adsorption behavior relative to water, methane, and n-hexane vapor. The studied compounds are characterized by reversibility of the changes in the crystal lattices during adsorption/desorption, stoichiometry of the reaction with water vapor with relatively low and hydrolytic stability at high humidity.


Similar content being viewed by others
References
D. Farrusseng (ed.), Metal–Organic Frameworks: Applications from Catalysis to Gas Storage, Wiley-VCH Verlag, Weinheim (2011).
L. R. MacGillivray, Metal–Organic Frameworks: Design and Application, Wiley-VCH Verlag, Hoboken (2010).
J.-K. Sun, M. Ji, C. Chen, et al., Chem. Commun., 49, No. 16, 1624–1626 (2013).
L. Yang, S. Kinoshita, T. Yamada, et al., Angew. Chem. Int. Ed., 49, No. 31, 5348–5351 (2010).
M. Yoon, K. Suh, S. Natarajan, and K. Kim, Angew. Chem. Int. Ed., 52, No. 10, 2688–2700 (2013).
H. Okawa, A. Shigematsu, M. Sadakiyo, et al., J. Am. Chem. Soc., 131, No. 37, 13516–13522 (2009).
I. L. Andriichuk, “Crystal structure, redox reactions, and sorption properties of metal-organic framework compounds based on carboxylate azamacrocyclic nickel(II) and copper(II) complexes,” Dissertation in competition for the academic degree of Candidate of Chemical Sciences, Kiev (2009).
T.-B. Lu, H. Xiang, R. L. Luck, et al., CrystEngComm., 3, No. 41, 168–169 (2001).
Ya. D. Lampeka and L. V. Tsymbal, Teor. Éksp. Khim., 40, No. 6, 331–356 (2004). [Theor. Exp. Chem., 40, No. 6, 345–371 (2004) (English translation).]
X.-R. Meng, D.-C. Zhong, L. Jiang, et al., Cryst. Growth Des., 11, No. 5, 2020–2025 (2011).
Ya. D. Lampeka, L. V. Tsymbal, A. V. Barna, et al., Dalton Trans., 41, No. 14, 4118–4125 (2012).
A. L. Spek, “PLATON: A Multipurpose Crystallographic Tool,” v. 1.16, Utrecht University, Utrecht, The Netherlands (2001).
S. Parsons, V. B. Jagaln, A. Harrison, et al., private communication (2006) (cited from search result in Cambridge Structural Database, version V5.34, structure code PELCOZ).
M. M. Dubinin, Abstracts, Sixth Conference on Theoretical Topics in Adsorption [in Russian], Nauka, Moscow (1985), pp. 141–148.
S. Noro, R. Kitaura, S. Kitagawa, et al., Inorg. Chem., 45, No. 22, 8990–8997 (2006).
C. E. Webster, R. S. Drago, and M. C. Zerner, J. Am. Chem. Soc., 120, No. 22, 5509–5516 (1998).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Teoreticheskaya i Éksperimental’naya Khimiya, Vol. 49, No. 4, pp. 255–261, July-August, 2013.
Rights and permissions
About this article
Cite this article
Tsymbal, L.V., Andriichuk, I.L., Yaremov, P.S. et al. Adsorption Properties of Porous Materials Based on Macrocyclic Nickel(II) Complexes and Benzenetricarboxylate Relative to Water, Methanol, and Hexane Vapor. Theor Exp Chem 49, 270–276 (2013). https://doi.org/10.1007/s11237-013-9326-6
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11237-013-9326-6