Abstract
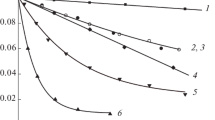
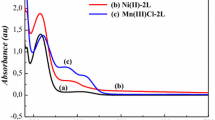
The kinetics of the decomposition of hydrogen peroxide in the presence of heterobimetallic complex compounds [M1(L1/L2)][M2Cl4] (M1=Ni, Cu; M2=Zn, Mn; L1=4,6,6-trimethyl-1,9-diamino-3,7-diaza-3-nonene); L2=1,15-dihydroxy-7,9,9-trimethyl-3,6,10,13-tetraaza-6-pentadecene), obtained by direct template synthesis, were studied. It was shown that the copper compounds have high catalytic activity, whereas the nickel complexes are inactive. A mathematical model is proposed for description of the kinetic relationships, and the main characteristics of the process are obtained.
Similar content being viewed by others
REFERENCES
G. A. Kovtun and I. I. Moiseev, Metal-Complex Inhibitors of Oxidation [in Russian], Naukova Dumka, Kiev (1993).
P. Zanello, S. Tamburini, P. A. Vigato, and G. A. Mazzocchin, Coord. Chem. Rev., 77, 165–273 (1987).
V. V. Skopenko, V. N. Kokozei, O. Yu. Vasil’eva, and S. R. Petrusenko, Teor. Éksp. Khim., 39, No.5, 265–274 (2003).
V. S. Kublanovskii, V. M. Kokozei, Yu. K. Pirskii, et al, “Heterometallic complexes [M(II)(L)][ZnCl 4 ] (M=Cu, Ni; L=4,6,6-trimethyl-1,9-diamino-3,7-diasa-3-nonene as precursors for preparation of electrocatalysts of oxygen reduction,“ Ukrainian Patent No. 71308 A, IPC H01 M4/90, H01 M4/92, Bull. No. 11, Publ. November 15, 2004.
N. A. Davidenko, V. N. Kokozei, D. V. Shevchenko, and I. I. Davidenko, Teor. Éksp. Khim., 40, No.1, 34–38 (2004).
V. M. Kokozei, D. V. Shevchenko, O. V. Pryma, S. P. Petrusenko, “The method for direct template synthesis of heterometallic complexes,” Ukrainian Patent No. 51007 A, IPC C 01 G1/00, Bull. No. 11, Publ. November 15, 2002.
I. V. Kudryashov (ed.), Practical Course of Physical Chemistry [in Russian], Vysshaya Shkola, Moscow (1986).
T. P. Vorob’eva and A. P. Purmal’, Zh. Fiz. Khim., 56, No.5, 1148–1153 (1982).
J. F. Perez-Benito, J. Inorg. Biochem., 98, 430–438 (2004).
R. Schmid and V. N. Sapunov, Nonformal Kinetics [Russian translation], Mir, Moscow (1985).
Matlab, The Language of Technical Computing, Version 5.3.0.10183 (R11), Copyright 1984–1999, The MathWorks Inc.
D. V. Shevchenko and V. S. Sudavtsova, Visn. Kyiv. Univ., 41, 20–21 (2004).
Author information
Authors and Affiliations
Additional information
__________
Translated from Teoreticheskaya i Éksperimental’naya Khimiya, Vol. 41, No. 1, pp. 17–23, January–February, 2005.
Rights and permissions
About this article
Cite this article
Diyuk, V.E., Shevchenko, D.V., Bezuglaya, T.N. et al. Catalytic activity of heterobimetallic M1/M2 complexes (M1=Ni, Cu; M2=Mn, Zn) in the decomposition of hydrogen peroxide. Theor Exp Chem 41, 19–25 (2005). https://doi.org/10.1007/s11237-005-0016-x
Received:
Issue Date:
DOI: https://doi.org/10.1007/s11237-005-0016-x