Abstract
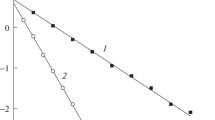
We have studied the effect of different surfactants on the rate of diethyl sulfide (Et2S) oxidation by hydrogen peroxide and peroxymonocarbonate (HCO -4 ) in aqueous solutions. In all the studied cases, the rate of the reaction between Et2S and H2O2 decreases as the surfactant concentration increases. The reaction of Et2S with HCO -4 is catalyzed by cationic surfactants and inhibited by neutral and anionic surfactants.
Similar content being viewed by others
REFERENCES
V. C. Yang, J. A. Baker, and J. R. Ward, Chem. Rev., 92, 1729–1743 (1992).
D. E. Richardson, H. Yao, K. M. Frank, and D. A. Bennett, J. Am. Chem. Soc., 122, No. 8, 1729–1739 (2000).
A. Blaschette, Z. Anorg. Allg. Chem., 384, No. 2, 177–183 (1971).
S. Vayssié and H. Elias, Angew. Chem., 37, No. 15, 2088–2090 (1998).
M. Chiarini, N. D. Gillitt, and C. A. Bunton, Langmuir, 18, 3836–3842 (2002).
C. A. Bunton and N. D. Gillitt, J. Phys. Org. Chem., 15, No. 1, 29–36 (2002).
B. Segues, E. Peres, J. Rico-Lattes, et al., Bull. Soc. Chim. France, 133, 925–937 (1996).
V. L. Lobachev, V. A. Savelova, and T. M. Prokop’eva, Teor. Éksp. Khim., 40, No. 3, 157–161 (2004).
Yu. S. Simanenko, E. A. Karpichev, T. M. Prokop’eva, et al., 40, No. 2, 234–246 (2004).
Weygand-Hilgentag, Preparative Organic Chemistry [Russian translation], Khimiya, Moscow (1969).
E. S. Rudakov, Reactions of Alkanes with Oxidizing Agents, Metal Complexes, and Radicals in Solutions [in Russian], Nauk. Dumka, Kiev (1985).
I. V. Berezin, K. Martinek, and A. K. Yatsimirskii, Usp. Khim., 42, No. 10, 1729–1756 (1973).
A. A. Abramzon and G. M. Gaevskii (eds.), Surfactants [in Russian], Khimiya, Leningrad (1979).
R. Bacaloglu, A. Blasko, C. A. Bunton, and H. Foroudian, J. Phys. Org. Chem., 5, No. 1, 171–178 (1992).
F. Ruff and A. Kucsman, J. Chem. Soc., Perkin Trans. II, No. 5, 683–687 (1985).
Author information
Authors and Affiliations
Additional information
Translated from Teoreticheskaya i Éksperimental’naya Khimiya, Vol. 40, No. 6, pp. 368–372, November–December, 2004.
Rights and permissions
About this article
Cite this article
Lobachev, V.L., Prokop’eva, T.M. & Savelova, V.A. Kinetics of diethyl sulfide oxidation by hydrogen peroxide and peroxymonocarbonate in the presence of surfactants. Theor Exp Chem 40, 383–388 (2004). https://doi.org/10.1007/s11237-005-0004-1
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/s11237-005-0004-1