Abstract
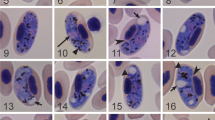
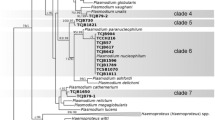
DNA barcoding (molecular characterisation) is a useful tool for describing the taxonomy and systematics of organisms. Over 250 species of avian haemosporidian parasites have been described using morphological characters, yet molecular techniques based on polymerase chain reaction (PCR) suggest this diversity is underestimated. Moreover, molecular techniques are particularly useful for the detection of chronic infections and tissue stages of these parasites. Species delimitation is problematic among haemosporidians, and many questions about the mechanisms and patterns of speciation, host specificity and pathogenicity are still unresolved. Accumulation of additional genetic and morphological information is needed to approach these questions. Here, we combine microscopic examination with PCR-based methods to develop molecular characterisation of Haemoproteus (Parahaemoproteus) manwelli Bennett, 1978 and Haemoproteus (Parahaemoproteus) gavrilovi Valkiūnas & Iezhova, 1990, both of which parasitise the bee-eater Merops apiaster L. We also describe a new species, Haemoproteus (Parahaemoproteus) palloris n. sp., from the blood of the willow warbler Phylloscopus trochilus (L.). We performed phylogenetic analyses with a set of mitochondrial cytochrome b (cyt b) gene lineages, which have been linked to parasite morphospecies and are available in the MalAvi database. Our findings show that morphological characters, which have been traditionally used in the description of haemosporidians, exhibit phylogenetic congruence. This study contributes to a better understanding of avian haemosporidian diversity and provides new molecular markers (cyt b and apicoplast gene sequences) for the diagnostics of inadequately investigated haemosporidian infections.



Similar content being viewed by others
References
Atkinson, C. T. (2008). Haemoproteus. In C. T. Atkinson, N. J. Thomas, & B. C. Hunter (Eds), Parasitic diseases of wild birds. Ames, IA: Wiley-Blackwell, pp. 13–35.
Bairlein, F. (1995). Manual of field methods. European-African songbird migration network. Institut für Vogelforschung, Wilhelmshaven.
Beadell, J. S., Covas, R., Gebhard, C., Ishtiaq, F., Melo, M., Schmidt, K., et al. (2009). Host associations and evolutionary relationships of avian blood parasites from West Africa. International Journal for Parasitology, 39, 257–266.
Beadell, J. S., Gering, E., Austin, J., Dumbacher, J. P., Peirce, M. A., Pratt, T. K., et al. (2004). Prevalence and differential host-specificity of two avian blood parasite genera in the Australo-Papuan region. Molecular Ecology, 13, 3829–3844.
Bennett, G. F. (1978). Avian Haemoproteidae. 8. The haemoproteids of the bee-eater family (Meropidae). Canadian Journal of Zoology, 56, 1721–1725.
Bennett, G. F., & Campbell, A. G. (1972). Avian Haemoproteidae. I. Description of Haemoproteus fallisi n. sp. and a review of the haemoproteids of the family Turdidae. Canadian Journal of Zoology, 50, 1269–1275.
Bensch, S., & Åkesson, S. (2003). Temporal and spatial variation of hematozoans in Scandinavian willow warblers. Journal of Parasitology, 89, 388–391.
Bensch, S., Hellgren, O., & Pérez-Tris, J. (2009). MalAvi: a public database of malaria parasites and related haemosporidians in avian hosts based on mitochondrial cytochrome b lineages. Molecular Ecology Resources, 9, 1353–1358.
Bensch, S., Pérez-Tris, J., Waldenström, J., & Hellgren, O. (2004). Linkage between nuclear and mitochondrial DNA sequences in avian malaria parasites: multiple cases of cryptic speciation? Evolution, 58, 1617–1621.
Bensch, S., Stjernman, M., Hasselquist, D., Ostman, O., Hansson, B., Westerdahl, H., & Pinheiro, R. T. (2000). Host specificity in avian blood parasites: a study of Plasmodium and Haemoproteus mitochondrial DNA amplified from birds. Proceedings of the Royal Society B: Biological Sciences, 267(1452), 1583–1589.
Bishop, M. A., & Bennett, G. F. (1989). The haemoproteids of the avian order Strigiformes. Canadian Journal of Zoology, 67, 2676–2684.
Bukauskaitė, D., Žiegytė, R., Palinauskas, V., Iezhova, T. A., Dimitrov, D., Ilgūnas, M., et al. (2015). Biting midges (Culicoides, Diptera) transmit Haemoproteus parasites of owls: evidence from sporogony and molecular phylogeny. Parasites & Vectors, 8, 303.
Cannell, B. L., Krasnec, K. V., Campbell, K., Jones, H. I., Miller, R. D., & Stephens, N. (2013). The pathology and pathogenicity of a novel Haemoproteus spp. infection in wild Little Penguins (Eudyptula minor). Veterinary Parasitology, 197, 74–84.
Clark, N. J., Adlard, R. D., & Clegg, S. M. (2015). Molecular and morphological characterization of Haemoproteus (Parahaemoproteus) ptilotis, a parasite infecting Australian honeyeaters (Meliphagidae), with remarks on prevalence and potential cryptic speciation. Parasitology Research, 114, 1921–1928.
Clark, N. J., Clegg, S. M., & Lima, M. R. (2014). A review of global diversity in avian haemosporidians (Plasmodium and Haemoproteus: Haemosporida): new insights from molecular data. International Journal for Parasitology, 44, 329–338.
Coral, A. A., Valkiūnas, G., González, A. D., & Matta, N. E. (2015). In vitro development of Haemoproteus columbae (Haemosporida: Haemoproteidae), with perspectives for genomic studies of avian haemosporidian parasites. Experimental Parasitology, 157, 163–169.
Cosgrove, C. L., Day, K. P., & Sheldon, B. C. (2006). Coamplification of Leucocytozoon by PCR diagnostic tests for avian malaria: a cautionary note. Journal of Parasitology, 92, 1362–1365.
Cosgrove, C. L., Wood, M. J., Day, K. P., & Sheldon, B. C. (2008). Seasonal variation in Plasmodium prevalence in a population of blue tits Cyanistes caeruleus. Journal of Animal Ecology, 77, 540–548.
Darriba, D., Taboada, G. L., Doallo, R., & Posada, D. (2012). jModelTest 2: more models, new heuristics and parallel computing. Nature Methods, 9, 772.
Dimitrov, D., Zehtindjiev, P., Bensch, S., Ilieva, M., Iezhova, T. A., & Valkiūnas, G. (2014). Two new species of Haemoproteus Kruse, 1890 (Haemosporida, Haemoproteidae) from European birds, with emphasis on DNA barcoding for detection of haemosporidians in wildlife. Systematic Parasitology, 87, 135–151.
Drovetski, S. V., Aghayan, S. A., Mata, V. A., Lopes, R. J., Mode, N. A., Harvey, J. A., & Voelker, G. (2014). Does the niche breadth or trade-off hypothesis explain the abundance-occupancy relationship in avian Haemosporidia? Molecular Ecology, 23, 3322–3329.
Fallon, S. M., Ricklefs, R. E., Swanson, B. L., & Bermingham, E. (2003). Detecting avian malaria: an improved Polymerase Chain Reaction diagnostic. Journal of Parasitology, 89, 1044–1047.
Feldman, R. A., Freed, L. A., & Cann, R. L. (1995). A PCR test for avian malaria in Hawaiian birds. Molecular Ecology, 4, 663–673.
Ferraguti, M., Martínez-de la Puente, J., Muñoz, J., Roiz, D., Ruiz, S., Soriguer, R., & Figuerola, J. (2013). Avian Plasmodium in Culex and Ochlerotatus mosquitoes from southern Spain: effects of season and host-feeding source on parasite dynamics. PLoS One, 8, e66237.
Godfrey, R. D., Fedynich, A. M., & Pence, D. B. (1987). Quantification of haematozoa in blood smears. Journal of Wildlife Diseases, 23, 558–565.
González, A. D., Lotta, I. A., García, L. F., Moncada, L. I., & Matta, N. E. (2015). Avian haemosporidians from Neotropical highlands: Evidence from morphological and molecular data. Parasitology International, 64, 48–59.
Greiner, E. C., Mandal, A. K., & Nandi, N. C. (1977). Haemoproteus bennetti sp. n. and a review of the Haemoproteus from Picidae. Journal of Parasitology, 63, 651–656.
Hall, T. (1999). BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symposium Series, 41, 95–98.
Hellgren, O., Atkinson, C. T., Bensch, S., Albayrak, T., Dimitrov, D., Ewen, J. G., et al. (2014). Global phylogeography of the avian malaria pathogen Plasmodium relictum based on MSP1 allelic diversity. Ecography, 38, 842–850.
Hellgren, O., Križanauskienė, A., Valkiūnas, G., & Bensch, S. (2007). Diversity and phylogeny of mitochondrial cytochrome B lineages from six morphospecies of avian Haemoproteus (Haemosporida: Haemoproteidae). Journal of Parasitology, 93, 889–896.
Hellgren, O., Kutzer, M., Bensch, S., Valkiūnas, G., & Palinauskas, V. (2013). Identification and characterization of the merozoite surface protein 1 (msp1) gene in a host-generalist avian malaria parasite, Plasmodium relictum (lineages SGS1 and GRW4) with the use of blood transcriptome. Malaria Journal, 12, 381.
Hellgren, O., Waldenström, J., & Bensch, S. (2004). A new PCR assay for simultaneous studies of Leucocytozoon, Plasmodium and Haemoproteus from avian blood. Journal of Parasitology, 90, 797–802.
Iezhova, T. A., Dodge, M., Sehgal, R. N. M., Smith, T. B., & Valkiūnas, G. (2011). New avian Haemoproteus species (Haemosporida: Haemoproteidae) from African birds, with a critique of the use of host taxonomic information in hemoproteid classification. Journal of Parasitology, 97, 682–694.
Ishtiaq, F., Gering, E., Rappole, J. H., Rahmani, A. R., Jhala, Y. V., Dove, C. J., et al. (2007). Prevalence and diversity of avian hematozoan parasites in Asia: a regional survey. Journal of Wildlife Diseases, 43, 382–398.
Jarvis, E. D., Mirarab, S., Aberer, A. J., Li, B., Houde, P., Li, C., et al. (2014). Whole-genome analyses resolve early branches in the tree of life of modern birds. Science, 346, 1320–1331.
Karadjian, G., Puech, M.-P., Duval, L., Chavatte, J.-M., Snounou, G., & Landau, I. (2013). Haemoproteus syrnii in Strix aluco from France: morphology, stages of sporogony in a hippoboscid fly, molecular characterization and discussion on the identification of Haemoproteus species. Parasite (Paris, France), 20, 32.
Križanauskienė, A., Hellgren, O., Kosarev, V., Sokolov, L., Bensch, S., & Valkiunas, G. (2006). Variation in host specificity between species of avian hemosporidian parasites: evidence from parasite morphology and cytochrome B gene sequences. Journal of Parasitology, 92, 1319–1324.
Križanauskienė, A., Iezhova, T. A., Sehgal, R. N. M., Carlson, J. S., Palinauskas, V., Bensch, S., & Valkiūnas, G. (2013). Molecular characterization of Haemoproteus sacharovi (Haemosporida, Haemoproteidae), a common parasite of columbiform birds, with remarks on classification of haemoproteids of doves and pigeons. Zootaxa, 3613, 85–94.
Križanauskienė, A., Pérez-Tris, J., Palinauskas, V., Hellgren, O., Bensch, S., & Valkiūnas, G. (2010). Molecular phylogenetic and morphological analysis of haemosporidian parasites (Haemosporida) in a naturally infected European songbird, the blackcap Sylvia atricapilla, with description of Haemoproteus pallidulus sp. nov. Parasitology, 137, 217–227.
Lauron, E. J., Oakgrove, K. S., Tell, L. A., Biskar, K., Roy, S. W., & Sehgal, R. N. M. (2014). Transcriptome sequencing and analysis of Plasmodium gallinaceum reveals polymorphisms and selection on the apical membrane antigen-1. Malaria Journal, 13, 382.
Li, J., Wirtz, R. A., McConkey, G. A., Sattabongkot, J., Waters, A. P., Rogers, M. J., & McCutchan, T. F. (1995). Plasmodium genus-conserved primers for species identification and quantitation. Experimental Parasitology, 81, 182–190.
Loiseau, C., Iezhova, T., Valkiūnas, G., Chasar, A., Hutchinson, A., Buermann, W., et al. (2010). Spatial variation of haemosporidian parasite infection in African rainforest bird species. Journal of Parasitology, 96, 21–29.
Mantilla, J. S., González, A. D., Valkiūnas, G., Moncada, L. I., & Matta, N. E. (2013). Description and molecular characterization of Plasmodium (Novyella) unalis sp. nov. from the Great Thrush (Turdus fuscater) in highland of Colombia. Parasitology Research, 112, 4193–4204.
Martínez, J., Martínez-de la Puente, J., Herrero, J., Del Cerro, S., Lobato, E., Rivero-de Aguilar, J., et al. (2009). A restriction site to differentiate Plasmodium and Haemoproteus infections in birds: on the inefficiency of general primers for detection of mixed infections. Parasitology, 136, 713–722.
Martinsen, E. S., Perkins, S. L., & Schall, J. J. (2008). A three-genome phylogeny of malaria parasites (Plasmodium and closely related genera): evolution of life-history traits and host switches. Molecular Phylogenetics and Evolution, 47, 261–273.
Matta, N. E., Pacheco, M. A., Escalante, A. A., Valkiūnas, G., Ayerbe-Quiñones, F., & Acevedo-Cendales, L. D. (2014). Description and molecular characterization of Haemoproteus macrovacuolatus n. sp. (Haemosporida, Haemoproteidae), a morphologically unique blood parasite of black-bellied whistling duck (Dendrocygna autumnalis) from South America. Parasitology Research, 113, 2991–3000.
Merino, S., Hennicke, J., Martínez, J., Ludynia, K., Torres, R., Work, T. M., et al. (2012). Infection by Haemoproteus parasites in four species of frigatebirds and the description of a new species of Haemoproteus (Haemosporida: Haemoproteidae). Journal of Parasitology, 98, 388–397.
Olias, P., Wegelin, M., Zenker, W., Freter, S., Gruber, A. D., & Klopfleisch, R. (2011). Avian malaria deaths in parrots, Europe. Journal of Infectious Diseases, 171, 950–952.
Ondrejicka, D. A., Locke, S. A., Morey, K., Borisenko, A. V., & Hanner, R. H. (2014). Status and prospects of DNA barcoding in medically important parasites and vectors. Trends in Parasitology, 30, 582–591.
Outlaw, D. C., & Ricklefs, R. E. (2014). Species limits in avian malaria parasites (Haemosporida): how to move forward in the molecular era. Parasitology, 2, 1–10.
Palinauskas, V., Iezhova, T. A., Križanauskienė, A., Markovets, M. Y., Bensch, S., & Valkiūnas, G. (2013a). Molecular characterization and distribution of Haemoproteus minutus (Haemosporida, Haemoproteidae): a pathogenic avian parasite. Parasitology International, 62, 358–363.
Palinauskas, V., Križanauskienė, A., Iezhova, T. A., Bolshakov, C. V., Jönsson, J., Bensch, S., & Valkiūnas, G. (2013b). A new method for isolation of purified genomic DNA from haemosporidian parasites inhabiting nucleated red blood cells. Experimental Parasitology, 133, 275–280.
Perkins, S. L., & Schall, J. J. (2002). A molecular phylogeny of malarial parasites recovered from cytochrome b gene sequences. Journal of Parasitology, 88, 972–978.
Poulin, R., & Keeney, D. B. (2008). Host specificity under molecular and experimental scrutiny. Trends in Parasitology, 24, 24–28.
Richard, F. A., Sehgal, R. N. M., Jones, H. I., & Smith, T. B. (2002). A Comparative Analysis of PCR-Based Detection Methods for Avian Malaria. Journal of Parasitology, 88, 819–822.
Richardson, D. S., Jury, F. L., Blaakmeer, K., Komdeur, J., & Burke, T. (2001). Parentage assignment and extra-group paternity in a cooperative breeder: the Seychelles warbler (Acrocephalus sechellensis). Molecular Ecology, 10, 2263–2273.
Ricklefs, R. E., & Fallon, S. M. (2002). Diversification and host switching in avian malaria parasites. Proceedings of the Royal Society B: Biological Sciences, 269, 885–892.
Ricklefs, R. E., Outlaw, D. C., Svensson-Coelho, M., Medeiros, M. C. I., Ellis, V. A., & Latta, S. (2014). Species formation by host shifting in avian malaria parasites. Proceedings of the National Academy of Sciences of the United States of America, 111, 14816–14821.
Ronquist, F., & Huelsenbeck, J. P. (2003). MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics, 19, 1572–1574.
Silveira, P., Belo, N. O., Lacorte, G. A., Kolesnikovas, C. K. M., Vanstreels, R. E. T., Steindel, M., et al. (2013). Parasitological and new molecular-phylogenetic characterization of the malaria parasite Plasmodium tejerai in South American penguins. Parasitology International, 62, 165–171.
Svensson, L. (1992). Identification Guide to European Passerines (4th ed., p. 368). Stockholm: British Trust for Ornithology.
Tamura, K., Stecher, G., Peterson, D., Filipski, A., & Kumar, S. (2013). MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Molecular biology and evolution, 30, 2725–2729.
Valkiūnas, G. (2005). Avian malaria parasites and other haemosporidia (p. 946). New: CRC PRESS Boca Raton.
Valkiūnas, G., Bensch, S., Iezhova, T. A., Križanauskienė, A., Hellgren, O., & Bolshakov, C. V. (2006). Nested cytochrome B polymerase chain reaction diagnostics underestimate mixed infections of avian blood haemosporidian parasites: microscopy is still essential. Journal of Parasitology, 92, 418–422.
Valkiūnas, G., Iezhova, T. A., Križanauskienė, A., Palinauskas, V., Sehgal, R. N. M., & Bensch, S. (2008a). A comparative analysis of microscopy and PCR-based detection methods for blood parasites. Journal of Parasitology, 94, 1395–1401.
Valkiūnas, G., Iezhova, T. A., Loiseau, C., Chasar, A., Smith, T. B., & Sehgal, R. N. M. (2008b). New species of haemosporidian parasites (Haemosporida) from African rainforest birds, with remarks on their classification. Parasitology Research, 103, 1213–1228.
Valkiūnas, G., Iezhova, T. A., Loiseau, C., & Sehgal, R. N. M. (2009). Nested cytochrome B polymerase chain reaction diagnostics detect sporozoites of hemosporidian parasites in peripheral blood of naturally infected birds. Journal of Parasitology, 95, 1512–1515.
Valkiūnas, G., Kazlauskienė, R., Bernotienė, R., Palinauskas, V., & Iezhova, T. A. (2013). Abortive long-lasting sporogony of two Haemoproteus species (Haemosporida, Haemoproteidae) in the mosquito Ochlerotatus cantans, with perspectives on haemosporidian vector research. Parasitology Research, 112, 2159–2169.
Valkiūnas, G., Križanauskienė, A., Iezhova, T. A., Hellgren, O., & Bensch, S. (2007). Molecular phylogenetic analysis of circumnuclear hemoproteids (Haemosporida: Haemoproteidae) of sylvid birds, with a description of Haemoproteus parabelopolskyi sp. nov. Journal of Parasitology, 93, 680–687.
Valkiūnas, G., Palinauskas, V., Ilgūnas, M., Bukauskaitė, D., Dimitrov, D., Bernotienė, R., et al. (2014). Molecular characterization of five widespread avian haemosporidian parasites (Haemosporida), with perspectives on the PCR-based detection of haemosporidians in wildlife. Parasitology Research, 113, 2251–2263.
Ventim, R., Morais, J., Pardal, S., Pérez-Tris, J., Ramos, J. A., & Mendes, L. (2012). Host-parasite associations and host-specificity in haemoparasites of reed bed passerines. Parasitology, 139, 310–316.
Waldenström, J., Bensch, S., Kiboi, S., Hasselquist, D., & Ottusson, U. (2002). Cross-species infection of blood parasites between resident and migratory songbirds in Africa. Molecular Biology and Evolution, 11, 1545–1554.
Waldenström, J., Bensch, S., & Ostman, O. (2004). A new nested polymerase chain reaction method very efficient in detecting Plasmodium and Haemoproteus infections from avian blood. Journal of Parasitology, 90, 191–194.
WHO. (2014). World Malaria Report 2014. Geneva. http://apps.who.int/iris/bitstream/10665/144852/2/9789241564830_eng.pdf?ua=1. Accessed 14 January 2015.
Wood, M. J., Childs, D. Z., Davies, A. S., Hellgren, O., Cornwallis, C. K., Perrins, C. M., & Sheldon, B. C. (2013). The epidemiology underlying age-related avian malaria infection in a long-lived host: the mute swan Cygnus olor. Journal of Avian Biology, 44, 12.
Zehtindjiev, P., Ivanova, K., Mariaux, J., & Georgiev, B. B. (2013). First data on the genetic diversity of avian haemosporidians in China: cytochrome b lineages of the genera Plasmodium and Haemoproteus (Haemosporida) from Gansu Province. Parasitology Research, 112, 3509–3515.
Acknowledgements
We are grateful to Karina Ivanova for her help during microscopic examination of material and to Strahil Peev, Konstantin Valchev, Stefan Braianavsci and Iovelina Bedeva for their help in the field work. This is report number 59 for Kalimok field station.
Funding
This study was supported by the European Union Structural Funds project “Postdoctoral Fellowship Implementation in Lithuania” (VP-3.1-ŠMM-01-V-02-004) and the postdoctoral project grant DO02-277 to MI from the Bulgarian Science Fund.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable institutional, national and international guidelines for the care and use of animals were followed. The research was conducted under permissions 213/26.06.2009 and 433/07.03.2012 issued from the Ministry of Evironment and Waters of Bulgaria.
Rights and permissions
About this article
Cite this article
Dimitrov, D., Iezhova, T.A., Zehtindjiev, P. et al. Molecular characterisation of three avian haemoproteids (Haemosporida, Haemoproteidae), with the description of Haemoproteus (Parahaemoproteus) palloris n. sp.. Syst Parasitol 93, 431–449 (2016). https://doi.org/10.1007/s11230-016-9638-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11230-016-9638-8