Abstract
In this paper, I show how idealizations contribute to social activities in science, such as the recruitment of experts to a research project. These contributions have not been explicitly discussed by recent philosophical accounts of scientific idealization (e.g., Potochnik in Idealization and the aims of science, University of Chicago Press, Chicago, 2017; Weisberg in Simulation and similarity: using models to understand the world, OUP, Oxford, 2013; Wimsatt in Re-engineering philosophy for limited beings: Piecewise approximations to reality, Harvard University Press, Cambridge, 2007). These accounts have focused on how idealizations influence activities like scientific theorization, explanation, and modeling. Other accounts focus on how idealizations influence policy-making and science communication (Gilbert and Justi in Modelling-based teaching in science education, Springer international publishing, Basel, Switzerland, 2016; Roussos in Philos Sci, 2020. https://doi.org/10.1086/712818). I expand these accounts by exploring the uses of idealized phylogenetic trees in science. Trees are not only useful for improving our understanding and public communication of evolutionary history, but they also help with the organization of laboratories and collaboration among scientists. Attending to the relation between idealizations and these social practices in science matters. It can help us understand why idealized models become entrenched and why certain social practices change over time.


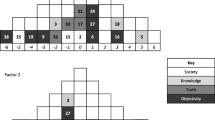
Source: adapted from Pardo et al. (2017)
Similar content being viewed by others
Notes
In this paper, I use the term ‘representation’ to refer to what philosophers of science usually treat as scientific representations, such as diagrams, equations, different types of models, but also theories and verbal descriptions or statements (Frigg and Nguyen 2016). Representations are things that can be deemed true, ‘true enough,’ false, veridical, accurate, inaccurate, or correct. In this sense, ‘representational activities’ are practices that directly result in these scientific representations. I explain terminological choice in the next section (also footnote 3).
Potochnik argues that the primary epistemic aim of science is understanding rather than truth. Her argument follows Catherine Elgin’s work (2004).
The label “representational activity” might cause confusion. In a basic sense, all activities involving models are representational. Models represent target systems and, thus, one must comprehend, interpret, manipulate, or extract information and content from these representations. In this sense, one can say that all uses of models are “representational activities” (I return to this point in Sect. 6). My thesis in this paper is that such uses often lead to the social organization of science. Yet, to make this thesis clearer, I employ “representational activities” when referring only to activities aiming at producing new scientific representations. Hence, my thesis can be re-stated as follows: models are often used in important social activities of scientists; activities that do not necessarily and directly generate new scientific representations.
Hence, the focus on truth implies the focus on representational activities but not vice-versa. One might argue that idealized models are useful for enabling scientists to arrive at new models, but this does not necessarily mean that such idealizations are enabling scientists to get the truth.
The group formed by the most recent common ancestor and all and only its descendants is called a monophyletic group (Hennig 1966; Wiley and Lieberman 2011). In Fig. 1, there are at least two monophyletic groups. The first one consists of A, B, and their most recent common ancestor, while the second consists of C, D, and their most recent common ancestor. Some phylogeneticists might also identify a third monophyletic group formed by every taxon (A, B, C, D) in the tree and their most recent common ancestor.
A welcome exception to this trend is Sterner and Lidgard (2018). This work analyzes the so-called “Systematics War”—the dispute between phylogenetic systematics and other schools of taxonomy in the second half of the twentieth Century. This analysis suggests that “systematists' theories about how to classify or infer evolutionary trees did not merely or even primarily aim to encapsulate representational knowledge about nature" (p. 33). According to this view, classification methods did not only contribute to the representation of knowledge, but also offered guidelines for producing and organizing this knowledge. Sterner and Lidgard (2018) focus on technical and procedural aspects in the production and organization of knowledge. In contrast, the present paper focuses on explicitly social and institutional aspects of this production and organization. I thank one of the reviewers for bringing this paper to my attention.
When biologists construct supertrees, they frequently use methods that involve pruning (Bininda-Emonds 2004, p. 316; Whidden et al. 2014). To prune a phylogenetic tree is to cut off some of its terminal branches or tips. When biologists prune a phylogenetic tree, they assume the isolation assumption. This assumption suggests that each terminal branch is attached to the tree only at one single point (its immediate common ancestor). Hence, the isolation assumption assures that, by pruning, one can cut a branch of the tree off and everything else will remain the same. This way of manipulating trees is relevant when combining source trees into supertrees.
In principle, the conflict between source trees might result from other things rather than conflicting molecular data sets. For example, different assumptions or method might generate conflicting trees. While Bininda-Emonds and other scholars seem aware of this fact, they are interested in occasions when the conflict between source trees results and is evidence of conflicting molecular data set.
Despite the similarity in the example, Woody’s contribution is different from mine in several respects. First and foremost, she proposes an account of explanation and argues that models (such as the periodic table) have an explanatory role. One of the roles would be guiding the creation of scientific norms and, thus, the social organization of scientific communities. In contrast, this paper is not committed to any account of explanation and does not argue that models must have an explanatory role in Woody’s sense. This paper explores the connection of models and practices more broadly, irrespective of whether one thinks these models explain or not (and what counts as explanation).
For examples, see the Mike Benton laboratory at the University of Bristol, the Per Ahlberg laboratory at the University of Uppsala, the Jason Anderson laboratory at the University of Calgary, and the Robert Reisz laboratory at the University of Toronto. For decades, Jenny Clack’s laboratory at the University of Cambridge was also important in this research (see Clack 2012).
To read about other aspects of this collaborative research, visit https://natureecoevocommunity.nature.com/users/51782-jason-s-anderson/posts/17990-datasets-revisited.
One might worry that my description of (3) relies on a problematic assumption about expertise. The assumption is that members of a laboratory become experts on specific parts of the phylogenetic trees rather than on animal groups independently from where these animals are on the tree. My description of (3) does not contain this assumption. Rather, I only assume that laboratories in vertebrate paleontology are well-known for working on animal groups in one or a few specific regions of the tree. Trees are convenient tools for identifying these groups.
The difference between a strictly branching phylogenetic tree and a network might be a matter of degree. If one includes one or two new branches connecting different parts of the tetrapod tree (Fig. 2), one will be turning a tree into a network. Nevertheless, networks typically contain many branches, which make them way less convenient for the activities (1)–(3) above.
References
Adl, S. M., et al. (2019). Revisions to the classification, nomenclature, and diversity of eukaryotes. Journal of Eukaryotic Microbiology, 66(1), 4–119.
Bapteste, E., & Huneman, P. (2018). Towards a dynamic interaction network of life to unify and expand the evolutionary theory. BMC Biology, 16, 1–16.
Batterman, R. W. (2002). Asymptotics and the role of minimal models. The British Journal for the Philosophy of Science, 53(1), 21–38.
Baum, D. A., & Smith, S. D. (2013). Tree thinking: An introduction to phylogenetic biology. Greenwood Village, CO: Roberts.
Benton, M. J. (2009). Vertebrate palaeontology. London: Wiley-Blackwell.
Bininda-Emonds, O. R. (2004). The evolution of supertrees. Trends in Ecology and Evolution, 19(6), 315–322.
Boto, L. (2014). Horizontal gene transfer in the acquisition of novel traits by metazoans. Proceedings of the Royal Society B. https://doi.org/10.1098/rspb.2013.2450
Burki, F., et al. (2020). The new tree of eukaryotes. Trends in Ecology and Evolution, 35(1), 43–55.
Cartwright, N. (1983). How the laws of physics lie. Oxford: OUP.
Cartwright, N. (1995). False idealisation: A philosophical threat to scientific method. Philosophical Studies, 77(2–3), 339–352.
Clack, J. A. (2012). Gaining ground the origin and evolution of tetrapods. Indiana: Indiana University Press.
Corel, E., et al. (2016). Network-thinking: Graphs to Analyze microbial complexity and evolution. Trends in Microbiology, 24, 224–237.
De Queiroz, K. (1999). The general lineage concept of species and the defining properties of the species category. In R. Wilson (Ed.), Species: Interdisciplinary essays (pp. 49–89). Massachusetts: MIT.
Elgin, C. Z. (2004). True enough. Philosophical Issues, 14, 113–131.
Ereshefsky, M. (1992). Eliminative pluralism. Philosophy of Science, 59(4), 671–690.
Ereshefsky, M. (2001). The poverty of the Linnaean hierarchy: A philosophical study of biological taxonomy. Cambridge: Cambridge University Press.
Frigg, R., & Nguyen, J. (2016). Scientific representation. The Stanford Encyclopedia of Philosophy. https://plato.stanford.edu/archives/spr2020/entries/scientific-representation. Accessed 02 April 2020.
Gee, B. M., Bevitt, J. J., & Reisz, R. R. (2019). Dissorophid diversity at the early Permian cave system near Richards Spur, Oklahoma, USA. Palaeontologia Electronica, 22, 1–32.
Giere, R. N. (1999). Using models to represent reality. In L. Magnani, N. Nersessian, & P. Thagard (Eds.), Model-based reasoning in scientific discovery (pp. 41–57). Berlin: Springer.
Gilbert, J. K., & Justi, R. (2016). Modelling-based teaching in science education (Vol. 9). Basel, Switzerland: Springer international publishing.
Hennig, W. (1966). Phylogenetic systematics. Chicago: University of Illinois Press.
Hinchliff, C. E., et al. (2015). Synthesis of phylogeny and taxonomy into a comprehensive tree of life. Proceedings of the National Academy of Sciences, 112(41), 12764–12769.
Huson, D. H., & Bryant, D. (2006). Application of phylogenetic networks in evolutionary studies. Molecular Biology and Evolution, 23(2), 254–267.
Huson, D. H., Rupp, R., & Scornavacca, C. (2010). Phylogenetic networks: Concepts, algorithms and applications. Cambridge: Cambridge University Press.
Kunin, V., et al. (2005). The net of life: Reconstructing the microbial phylogenetic network. Genome Research, 15(7), 954–959.
Lee, S. C., et al. (2008). Microsporidia evolved from ancestral sexual fungi. Current Biology: CB, 18(21), 1675–1679.
Levins, R. (1966). The strategy of model building in population biology. American Scientist, 54(4), 421–431.
Mallet, J. (2005). Hybridization as an invasion of the genome. Trends in Ecology and Evolution, 20(5), 229–237.
Mallet, J. (2007). Hybrid speciation. Nature, 446(7133), 279.
McInerney, J. O., & Erwin, D. H. (2017). The role of public goods in planetary evolution. Philosophical Transactions of A Mathematical Physical and Engineering, 375, 20160359.
Morgan, M. S., & Morrison, M. (1999). Models as mediators: Perspectives on natural and social science. Cambridge: Cambridge University Press.
Morrison, H. G., et al. (2007). Genomic minimalism in the early diverging intestinal parasite Giardia lamblia. Science, 317(5846), 1921–1926.
Neto, C. (2019). What is a lineage? Philosophy of Science, 86(5), 1099–1110.
Neto, C. (2020). When imprecision is a good thing, or how imprecise concepts facilitate integration in biology. Biology and Philosophy, 35(6), 1–21.
Pardo, J. D., et al. (2017). Hidden morphological diversity among early tetrapods. Nature, 546(7660), 642.
Potochnik, A. (2017). Idealization and the aims of science. Chicago: University of Chicago Press.
Ragan, M. A. (2001). On surrogate methods for detecting lateral gene transfer. FEMS Microbiology Letters, 201(2), 187–191.
Ravenhall, M., Škunca, N., Lassalle, F., & Dessimoz, C. (2015). Inferring horizontal gene transfer. PLoS Computational Biology, 11(5), e1004095.
Rohwer, Y., & Rice, C. (2013). Hypothetical pattern idealization and explanatory models. Philosophy of Science, 80(3), 334–355.
Roussos, J., Bradley, R., & Frigg, R. (2020). Making confident decisions with model ensembles. Philosophy of Science. https://doi.org/10.1086/712818
Sanderson, M. J., Purvis, A., & Henze, C. (1998). Phylogenetic supertrees: Assembling the trees of life. Trends in Ecology and Evolution, 13(3), 105–109.
Sober, E. (1991). Reconstructing the past: Parsimony, evolution, and inference. MIT press.
Sterner, B., & Lidgard, S. (2018). Moving past the systematics wars. Journal of the History of Biology, 51(1), 31–67.
Strevens, M. (2008). Depth: An account of scientific explanation. Cambridge: Harvard University Press.
Timmis, J. N., et al. (2004). Endosymbiotic gene transfer: Organelle genomes forge eukaryotic chromosomes. Nature Reviews Genetics, 5(2), 123.
Tripp, E. A., et al. (2017). Reshaping Darwin’s tree: Impact of the symbiome. Trends in Ecology and Evolution, 32, 552–555.
Velasco, J. D. (2012). The future of systematics: Tree thinking without the tree. Philosophy of Science, 79(5), 624–636.
Waters, C. K. (2014). Shifting attention from theory to practice in philosophy of biology. In M. C. Galavotti, D. Dieks, W. J. Gonzalez, S. Hartmann, T. Uebel, & M. Weber (Eds.), New directions in the philosophy of science (pp. 121–139). Dordrecht: Springer.
Waters, C. K. (2019). Presidential address, PSA 2016: An epistemology of scientific practice. Philosophy of Science, 86(4), 585–611.
Weisberg, M. (2007). Three kinds of idealization. The Journal of Philosophy, 104(12), 639–659.
Weisberg, M. (2013). Simulation and similarity: Using models to understand the world. Oxford: OUP.
Whidden, C., Zeh, N., & Beiko, R. G. (2014). Supertrees based on the subtree prune-and-regraft distance. Systematic Biology, 63(4), 566–581.
Wiley, E. O., & Lieberman, B. S. (2011). Phylogenetics: Theory and practice of phylogenetic systematics. New York: Wiley.
Wimsatt, W. C. (1987). False models as means to truer theories. In M. H. Nitecki & A. Hoffman (Eds.), Neutral models in biology (pp. 23–55). Oxford: Oxford University Press.
Wimsatt, W. C. (2007). Re-engineering philosophy for limited beings: Piecewise approximations to reality. Cambridge: Harvard University Press.
Woody, A. I. (2014). Chemistry’s periodic law: Rethinking representation and explanation after the turn to practice. In L. Soler, S. Zwart, M. Lynch, & V. Israel-Jost (Eds.), Science after the practice turn in the philosophy, history, and social studies of science (pp. 123–150). London: Routledge.
Zhaxybayeva, O., & Doolittle, W. F. (2011). Lateral gene transfer. Current Biology, 21(7), R242–R246.
Acknowledgements
I thank Marc Ereshefsky, Angela Potochnik, Ken Waters, and the audience at POBAMz 2020 for helpful feedback on early versions of this work. I thank Lorena Machado for the figures. The Izaak Waltom Killam Memorial Scholarship funded part of this research during the final year of my PhD at the University of Calgary. Finally, I thank Ford Doolittle and Letitia Meynell for my Postdoctoral Fellowship at the Dalhousie University.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Neto, C. From idealizations to social practices in science: the case of phylogenetic trees. Synthese 199, 10865–10884 (2021). https://doi.org/10.1007/s11229-021-03271-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11229-021-03271-9