Abstract
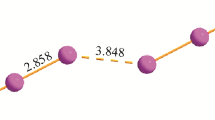
The crystal structure and properties of (Z)-4-chloro-5-((2-((4-chloro-5H-1,2,3-dithiazol-5-ylidene)amino)phenyl)amino)-1,2,3-dithiazol-1-ium oligoiodide (C2/c) synthesized from the initial bis(4-сhloro-5H-1,2,3-dithiazolo-5-ylidene)benzene-1,2-diamine (P21/c) have been characterized by various experimental and theoretic methods. The superposition of atomic basin boundaries in the electron density and in the electrostatic potential does not confirm the halogen bonding between the triiodide anion and sulfur atoms in cation. On the other hand, in the studied oligoiodide, the charge-assisted iodine–iodine halogen bonds are observed between the strongly asymmetric triiodide and diiodine units; thus, the oligoiodide anion includes at least two diiodine fragments with bond lengths 2.7334(4) and 2.7786(5) Å bound. This key trait has resulted in characteristic spectral and thermal features. Raman spectra do not contain typical triiodide bands but only those that are expectable for bound diiodine at 157 and 179 cm−1. Thermal decomposition has demonstrated release of both diiodine molecules within one decomposition stage without melting.






Similar content being viewed by others
References
Yu H, Yan L, He Y, Meng H, Huang W (2017) Chem Commun 53:432–435
Yin Z, Wang Q-X, Zeng M-H (2012) J Am Chem Soc 134:4857–4863
Svensson PH, Kloo L (2003) Chem Reviews 103(5):1649–1684
Desiraju GR, Ho PS, Kloo L (2013) Pure Appl Chem 85(8):1711–1713
Blake AJ, Devillanova FA, Gould RO, Li W-S, Lippolis V, Parsons S, Radek C, Schroder M (1998) Chem Soc Rev 27:195–205
Beno BR, Yeung K-S, Bartberger MD, Pennington LD, Meanwell NA (2015) J Med Chem 58(11):4383–4438
Shibaeva RP, Yagubskii EB (2004) Chem Rev 104:5347–5378
Rakitin OA (2011) Russ Chem Rev 80:647–659
Konstantinova LS, Rakitin OA (2008) Russ Chem Rev 77:521–546
Rawson JM, Alberola A, Whalley A (2006) J Mater Chem 16:2560–2575
Barclay TM, Cordes AW, Goddard JD, Mawhinney RC, Oakley RT, Preuss KE, Reed RW (1997) J Am Chem Soc 119:12136–12141
Barclay TM, Cordes AW, Oakley RT, Preuss KE, Reed RW (1998) Chem Commun:1039–1040
Barclay TM, Beer L, Cordes AW, Oakley RT, Preuss KE, Reed RW, Taylor NJ (2001) Inorg Chem 40:2709–2714
Wang Y-H, Lu Y-X, Zou J-W, Yu Q-S (2008) Int J Quantum Chem 108:90–99
Shi Q-C, Lu Y-X, Fan J-C, Zou J-W, Wang Y-H (2008) J Mol Struct 853:39–44
Clark T, Hennemann M, Murray JS, Politzer P (2007) J Mol Model 13:291–296
Politzer P, Riley KE, Bulat FA, Murray JS (2012) Comput Theor Chem 998:2–8
Groenewald F, Esterhuysen C, Dillen J (2012) Theor Chem Accounts 131:1–12
Bartashevich EV, Matveychuk YV, Troitskaya EA, Tsirelson VG (2014) Computational and Theoretical Chemistry 1037:53–62
Desiraju GR, Parthasarathy R (1989) J Am Chem Soc 111:8725–8726
Bartashevich EV, Shmanina EA, Yushina ID, Tsirelson VG, Kim DG (2014) J Struct Chem 55:154–160
Bartashevich EV, Batalov VI, Yushina ID, Stash AI, Chen YS (2016) Acta Crystallographica Section C 72:341–345
Konstantinova LS, Rakitin OA, Rees CW, Sivadasan S, Torroba T (1998) Tetrahedron 54:9639–9650
Sheldrick GM (2008) Acta Cryst A 64:112–122
The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif
Dovesi R, Orlando R, Erba A, Zicovich-Wilson CM, Civalleri B, Casassa S, Maschio L, Ferrabone M, De La Pierre M, D’Arco P, Noel Y, Causa M, Rerat M, Kirtman B (2014) Int J Quantum Chem 114:1287–1317
Lee C, Yang W, Parr RG (1988) Phys Rev B37:785–789
Becke AD (1988) Phys Rev 38:3098–3100
Iodine Basis set. URL: http://www.tcm.phy.cam.ac.uk/~mdt26/basis_sets/I_basis.txt
Gatti C, Saunders VR, Roetti CJ (1994) J Chem Phys 101:10686–10696
Maschio L, Kirtman B, Rerat M, Orlando R, Dovesi R (2013) J Chem Phys 139:164101
Silvi B, Savin A (1994) Nature 371:683–686
Gatti C, Casassa S (2016) Topond14. User’s Manual. http://www.istm.cnr.it/csrsrc/sw_topond.html
I. Yusina – S. Casassa personal communication, 2017.
Granovsky AA Firefly version 8. http://classic.chem.msu.su/gran/firefly/index.html
Lu T, Chen F (2012) J Comput Chem 33:580–592
Hübschle CB, Dittrich B (2011) J Appl Crystallogr 44:238–257
Hübschle CB, Luger P (2006) J Appl Crystallogr 39:901–904
Bartashevich EV, Stash AI, Batalov VI, Yushina ID, Drebushchak TN, Boldyreva EV, Tsirelson VG (2016) Struct Chem 27:1553–1560
Aragoni MC, Arca M, Caltagirone C, Castellano C, Demartin F, Garau A, Isaia F, Lippolis V, Montisc R, Pintus A (2012) Cryst Eng Comm 14:5809–5823
Groom CR, Bruno IJ, Lightfoot MP, Ward SC (2016) Acta Cryst B72:171–179
Cameron TS, Decken A, Fang M, Parsons S, Passmore J, Wood DJ (1999) Chem Commun:1801–1802
Beer L, Cordes AW, Haddon RC, Itkis ME, Oakley RT, Reed RW, Robertson CM (2002) Chem Commun:1872–1873
Barclay TM, Beer L, Cordes AW, Oakley RT, Preuss KE, Taylor NJ, Reed RW (1999) Chem Commun:531–532
Bader RFW (1990) Atoms in molecules. A quantum theory. Oxford University Press, New York,
Abate A, Brischetto M, Cavallo G (2010) Chem Commun 46:2724
Nelyubina YV, Antipin MY, Dunin DS (2010) Chem Commun 46:5325–5327
Megen M, Reiss GJ (2013) Inorganics 1:3–13
Tebbe K-F, Loukili RZ (1998) Anorg Allg Chem 624:1175
Gaballa AS, Teleb SM, Rusanov E, Steinborn D (2004) Inorg Chim Acta 357:4144
Giese M, Albrecht M, Bohnen C, Repenko T, Valkonen A, Rissanen K (2014) Dalton Trans 43:1873
Batalov VI, Kim DG, Dikhtiarenko A, Amghouz Z, Bartashevich EV, Garcia-Granda S (2014) Z Kristallogr New Cryst Struct 229:211–212
Bartashevich EV, Yushina ID, Vershinina EA, Slepukhin PA, Kim DG (2014) J Struct Chem 55:112–119
Batsanov AS, Bryce MR, Chesney A, Howard JAK, John DE, Moore AJ, Wood CL, Gershtenman H, Becker JY, Khodorkovsky VY, Ellern A, Bernstein J, Perepichka IF, Rotello V, Gray M, Cuello AO (2001) J Mater Chem 11:2181
Murata T, Morita Y, Yakiyama Y, Fukui K, Yamochi H, Saito G, Nakasuji K (2007) J Am Chem Soc 129:10837
Warden AC, Warren M, Hearn MTW, Spiccia L (2004) New J Chem 28:1160
Bartashevich EV, Yushina ID, Stash AI, Tsirelson VG (2014) Cryst Growth Des 14:5674–5684
Mata I, Molins E, Alkorta I, Espinosa E (2007) J Phys Chem A 111:6425–6433
Shishkina AV, Stash AI, Civalleri B, Ellern A, Tsirelson VG (2010) Mendeleev Commun 20:161–164
Mata I, Alkorta I, Molins E, Espinosa E (2013) Chem Phys Lett 555:106–109
Bader RFW, Beddall P (1972) J Chem Phys 56:3320–3329
Tsirelson VG, Shishkina AV, Stash AI, Parsons S (2009) Acta Crystallogr B65:647–658
Deplano P, Ferraro JR, Mercuri ML, Trogu EF (1999) Coord Chem Rev 188:71–95
Arca M, Aragoni MC, Devillanova FA, Garau A, Isaia F, Lippolis V, Mancini A, Verani G (2006) Bioinorganic Chemistry and Applications Article ID 58937:1–12
Yushina I, Rudakov B, Krivtsov I, Bartashevich E (2014) J Therm Anal Calorim 118:425–429
Acknowledgements
This work was supported by the Ministry of Education and Science of the Russian Federation, grant 4.1157.2017/PP and the Russian Foundation for Basic Research, grant No. 17-03-00406. O.I.B. and O.A.R. are grateful for the financial support from the Russian Science Foundation, grant No. 15-13-10022. The authors express their gratitude to Sylvia Casassa for the assistance in specifying computations with the use of TOPOND14.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
ESM 1
(PDF 537 kb)
Rights and permissions
About this article
Cite this article
Bol’shakov, O., Yushina, I., Bartashevich, E. et al. Asymmetric triiodide-diiodine interactions in the crystal of (Z)-4-chloro-5-((2-((4-chloro-5H-1,2,3-dithiazol-5-ylidene)amino)phenyl)amino)-1,2,3-dithiazol-1-ium oligoiodide. Struct Chem 28, 1927–1934 (2017). https://doi.org/10.1007/s11224-017-0987-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-017-0987-y