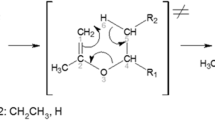
Abstract
An experimental study of the thermal decomposition of a β-hydroxy alkene, 3-buten-1-ol, in m-xylene solution, has been carried out at three different temperatures: 553.15, 573.15, and 593.15 K. The temperature dependence of the rate constants for the decomposition of this compound in the corresponding Arrhenius equation is given by ln k (s−1) = (27.34 ± 1.24)–(19,328 ± 712) (kJ mol−1) T −1. A computational study has been performed at the MP2/6-31+G(d) level of theory to calculate the rate constants and the activation parameters by the classical transition state theory. The Arrhenius equation obtained theoretically, ln k (s−1) = (28.252 ± 0.025)–(19,738.0 ± 14.4) (kJ mol−1) T −1, agrees very satisfactorily with the experimental one. The bonding characteristics of reactant, transition state, and products have been investigated by the natural bond orbital analysis which provides the natural atomic charges and the Wiberg bond indices used to follow the progress of the reaction. The enthalpy of the reaction has been calculated using experimental values taken from literature and theoretic calculations. The agreement between both values is satisfactory.





Similar content being viewed by others
References
Smith GG, Yates BL (1965) J Chem Soc 7242-7246
Arnold RT, Smolinsky G (1960) J Org Chem 25:129-130
Smith GG, Taylor R (1961) Chem Ind 949-950
Quijano J, David J, Sánchez C, Rincón E, Guerra D, León LA, Notario R, Abboud JL (2002) J Mol Struct (Theochem) 580:201–205
DePuy CH, King RW (1960) Chem Rev 60:431–457
Smith GG, Blau SE (1964) J Phys Chem 68:1231–1234
Zapata E, Gaviria J, Quijano J (2007) Int J Chem Kinet 39:92–96
Murillo J, Henao D, Vélez E, Castaño C, Quijano J, Gaviria J, Zapata E (2012) Int J Chem Kinet 44:407–413
Glasstone KJ, Laidler KJ, Eyring H (1941) The theory of rate processes, Chap 4. McGraw-Hill, New York
Benson SW (1969) The foundations of chemical kinetics. McGraw-Hill, New York
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery Jr JA, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas Ö, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian 09, Revision A.1, Gaussian Inc., Wallingford
Møller C, Plesset M (1934) Phys Rev 46:618–622
Merrick JP, Moran D, Radom L (2007) J Phys Chem A 111:11683–11700
McQuarrie DA, Simon JD (1999) Molecular thermodynamics. University Science Books, Sausalito
Fukui K (1970) J Phys Chem 74:4161–4163
Tomasi J, Mennucci B, Cammi R (2005) Chem Rev 105:2999–3094
Wohlfarth C (1955) In: Lide RD (ed) CRC handbook of chemistry and physics. CRC, Boca Raton, pp 185–199
Reed AE, Weinhold F (1983) J Chem Phys 78:4066–4073
Reed AE, Curtiss LA, Weinhold F (1988) Chem Rev 88:899–926
Wiberg KB (1968) Tetrahedron 24:1083–1096
Glendening ED, Reed AE, Carpenter JE, Weinhold F (1988) NBO Version 3.1, Madison
Moyano A, Pericàs MA, Valentí E (1989) J Org Chem 54:573–582
Vélez E, Quijano J, Gaviria J, Roux MV, Jiménez P, Temprado M, Martín-Valcárcel G, Pérez-Parajón J, Notario R (2005) J Phys Chem A 109:7832–7838
Pedley JB (1994) Thermochemical data and structures of organic compounds, vol. 1, TRC data series, TRC, College Satation
Acknowledgments
This study is supported by the research funds provided by Universidad Nacional de Colombia, Project “201010011033”, “Estudio Computacional y Experimental de la Eliminación de 3-Metil-3-buten-1-ol en Solución de m-Xileno,” DIME 2012, Modalidad 2.” R.N. thanks the financial support of the Spanish Ministerio de Economía y Competitividad under Project CTQ2010-16402.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is dedicated to María Victoria Roux on the occasion of her retirement.
Rights and permissions
About this article
Cite this article
López, V., Quijano, J., Luna, S. et al. Experimental and computational study of the thermal decomposition of 3-buten-1-ol in m-xylene solution. Struct Chem 24, 1811–1816 (2013). https://doi.org/10.1007/s11224-013-0234-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-013-0234-0