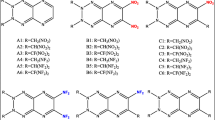
Abstract
The series of nitro-triaziridines had been studied as high-energy density compounds at B3LYP/6-311G** and MP2/6-311G** levels by means of density functional theory. The heats of formation (HOFs), bond dissociation energies, and detonation performance had been calculated in detail. It was found that all nitro-triaziridines have high position HOFs, and electron-withdrawing of nitro, the steric hindrance, and abundant N–N bond had positive effect with increasing values of HOFs. The thermodynamic stability is estimated by bond dissociation energy and available free space per molecule in unit cell. The detonation performance had been estimated via Kamlet–Jacobs equation and relative specific, However, two different consequences were obtained for detonation performance. Hence, for nitro-triaziridines derivatives, we assumed that a large number of extra oxygen was produced in combustion reaction or explosive reaction, which was negative for the energy released. Therefore, the oxygen balance must be considered for designing high-energy compounds. We also assumed that the Kamlet–Jacobs equation may not be applicable for the compounds, which was constituted of only oxygen, hydrogen, and nitrogen elements.

Similar content being viewed by others
References
Hammerl A, Klapötke TM (2002) Tetrazolylpentazoles: nitrogen-rich compounds. Inorg Chem 41(4):906–912
Sikder AK, Sikder N (2004) A review of advanced high performance, insensitive and thermally stable energetic materials emerging for military and space applications. J Hazard Mater 112(1–2):1–15
Strout DL (2003) Stabilization of an all-nitrogen molecule by oxygen insertion: dissociation pathways of N8O6. J Phys Chem A 107(10):1647–1650
Liu Y, Gong X, Wang L, Wang G, Xiao H (2011) Substituent effects on the properties related to detonation performance and sensitivity for 2,2′,4,4′,6,6′-hexanitroazobenzene derivatives. J Phys Chem A 115(9):1754–1762
Wang GX, Gong XD, Du HC, Liu Y, Xiao HM (2011) Theoretical prediction of properties of aliphatic polynitrates. J Phys Chem A 115(5):795–804
Korkin AA, Bartlett RJ (1996) Theoretical prediction of 2,4,6-trinitro-1,3,5-triazine (TNTA). A new, powerful, high-energy density material? J Am Chem Soc 118(48):12244–12245
Leininger ML, Sherrill CD, Schaefer HF III (1995) N8: a structure analogous to pentalene, and other high-energy density minima. J Phys Chem 99(8):2324–2328
Yarkony DR (1992) Theoretical studies of spin-forbidden radiationless decay in polyatomic systems: insights from recently developed computational methods. J Am Chem Soc 114(13):5406–5411
Yildirim T, Gehring PM, Neumann DA, Eaton PE, Emrick T (1997) Unusual structure, phase transition, and dynamics of solid cubane. Phys Rev Lett 78(26):4938–4941
Leininger ML, Van Huis TJ, Schaefer HF (1997) Protonated high energy density materials: N4 tetrahedron and N8 octahedron. J Phys Chem A 101(24):4460–4464
Huynh MHV, Hiskey MA, Chavez DE, Naud DL, Gilardi RD (2005) Synthesis, characterization, and energetic properties of diazido heteroaromatic high-nitrogen C–N compound. J Am Chem Soc 127(36):12537–12543
Miller DR, Swenson DC, Gillan EG (2004) Synthesis and structure of 2,5,8-triazido-s-heptazine: an energetic and luminescent precursor to nitrogen-rich carbon nitrides. J Am Chem Soc 126(17):5372–5373
Ciezak JA, Trevino SF (2005) The inelastic neutron scattering spectra of α-3-amino-5-nitro-1,2,4–2H-triazole: experiment and DFT calculations. Chem Phys Lett 403(4–6):329–333
Talawar MB, Sivabalan R, Senthilkumar N, Prabhu G, Asthana SN (2004) Synthesis, characterization and thermal studies on furazan- and tetrazine-based high energy materials. J Hazard Mater 113(1–3):11–25
Chi WJ, Li LL, Li BT, Wu HS (2012) Density functional study on the derivatives of purine. J Mol Model. doi:10.1007/s00894-012-1359-6
David WB (2002) High-level ab initio calculations on hydrogen–nitrogen compounds. Thermochemistry of tetrazetidine, N4H4. J Mol Struct Theochem 619(1–3):37–43
Chi WJ, Li LL, Li BT, Wu HS (2012) Density functional calculation on a high energy density compound having the formula C2OH4-n (NO2)n. Struct Chem. doi:10.1007/s11224-012-9992-3
Nguyen M-T, Kaneti J, Hoesch L, Dreiding AS (1984) Triaziridines. Part III. Triaziridine, azimine, and triazene: a SCF study of the energy and structure of N3H3-isomers. Helv Chim Acta 67(7):1918–1929
Magers DH, Salter EA, Bartlett RJ, Salter C, Hess BA, Schaad LJ (1988) Do stable isomers of N3H3 exist? J Am Chem Soc 110(11):3435–3446
Zhao M, Gimarc BM (1994) Strain Energies of (NH)n Rings, n = 3–8. J Phys Chem 98(31):7497–7503
Inagaki S, Ishitani Y, Kakefu T (1994) Geminal delocalization of sigma—electrons and ring strains. J Am Chem Soc 116(13):5954–5958. doi:10.1021/ja00092a052
Zeigarnik AV, Valdés-Pérez RE (1998) Systematic prediction of the products and intermediates of isotopic labeling in reaction pathway studies. J Comput Chem 19(7):741–753
Glukhovtsev MN, Bach RD, Laiter S (1997) High-level computational study on the thermochemistry of saturated and unsaturated three- and four-membered nitrogen and phosphorus rings. Int J Quantum Chem 62(4):373–384
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Montgomery JA Jr, Vreven T, Kudin KN, Burant JC, Millam JM, Iyengar SS, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson GA, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox JE, Hratchian HP, Cross JB, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Ayala PY, Morokuma K, Voth GA, Salvador P, Dannenberg JJ, Zakrzewski VG, Dapprich S, Daniels AD, Strain MC, Farkas O, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Ortiz JV, Cui Q, Baboul AG, Clifford S, Cioslowski J, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Gonzalez C, Pople JA (2004) Gaussian 03 revision C 02. Gaussian, Inc., Wallingford
Becke AD (1988) Density-functional exchange-energy approximation with correct asymptotic behavior. Phys Rev A 38(6):3098–3100
Lee C, Yang W, Parr RG (1988) Development of the Colle–Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B 37(2):785–789
Møller C, Plesset MS (1934) Note on an approximation treatment for many-electron systems. Phys Rev 46(7):618–622
Wei T, Zhu W, Zhang J, Xiao H (2010) DFT study on energetic tetrazolo-[1,5-b]-1,2,4,5-tetrazine and 1,2,4-triazolo-[4,3-b]-1,2,4,5-tetrazine derivatives. J Hazard Mater 179(1–3):581–590
FJO (1996) Calculation of energy barriers for bond rupture in some energetic molecules. J Mol Struct Theochem 370 (1):11–16
Blanksby SJ, Ellison GB (2003) Bond dissociation energies of organic molecules. Acc Chem Res 36(4):255–263
Kamlet MJ, Jacobs SJ (1968) Chemistry of detonations. I. A simple method for calculating detonation properties of C–H–N–O explosives. J Chem Phys 48(1):23–35
Rice BM, Hare JJ, Byrd EFC (2007) Accurate predictions of crystal densities using quantum mechanical molecular volumes. J Phys Chem A 111(42):10874–10879
Politzer P, Martinez J, Murray JS, Concha MC, Toro-Labbé A (2009) An electrostatic interaction correction for improved crystal density prediction. Mol Phys 107(19):2095–2101
Bulat F, Toro-Labbé A, Brinck T, Murray J, Politzer P (2010) Quantitative analysis of molecular surfaces: areas, volumes, electrostatic potentials and average local ionization energies. J Mol Model 16(11):1679–1691
Politzer P, Murray JS, Grice ME, Sjoberg P (1991) Chemistry of energetic materials. Academic Press, San Diego, p 1991
Meyer R (1987) Explosives, 3rd ed. Weinheim: 71, VCH
Fan X-W, Ju X-H (2008) Theoretical studies on four-membered ring compounds with NF2, ONO2, N3, and NO2 groups. J Comput Chem 29(4):505–513
Pospíšil M, Vávra P, Concha M, Murray J, Politzer P (2010) A possible crystal volume factor in the impact sensitivities of some energetic compounds. J Mol Model 16(5):895–901
Pospíšil M, Vávra P, Concha M, Murray J, Politzer P (2011) Sensitivity and the available free space per molecule in the unit cell. J Mol Model 17(10):2569–2574
Keshavarz MH, Pouretedal HR (2005) Simple empirical method for prediction of impact sensitivity of selected class of explosives. J Hazard Mater 124(1–3):27–33
Richard RM, Ball DW (2009) Density functional calculations on the thermodynamic properties of a series of nitrosocubanes having the formula C8H8−x(NO)x (x = 1–8). J Hazard Mater 164(2–3):1552–1555
Politzer P, Murray JS (2011) Cent Eur J Energ Mater 8(3):209–220
Acknowledgments
This study is supported by the Natural Science Foundation of Shanxi Province (No. 2010021009-2), the Natural Science Foundation of China (No. 20871077), the Research Project Supported by Shanxi Scholarship Council of China (No. 201063), and the Natural Science Foundation of Shanxi Normal University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chi, W., Li, B. & Wu, H. Density function theory study on energetic nitro-triaziridine derivatives. Struct Chem 24, 375–381 (2013). https://doi.org/10.1007/s11224-012-0083-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-012-0083-2