Abstract
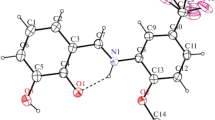
The Schiff base compound (E)-2-(1-(2-(4-methylphenylsulfonamido)ethyliminio)ethyl) phenolate has been synthesised and characterized by IR, UV–Vis, and X-ray single-crystal determination. Ab initio calculations have been carried out for the title compound using the density functional theory (DFT) and Hartree–Fock (HF) methods at 6-31G(d) basis set. The calculated results show that the DFT/B3LYP and HF can well reproduce the structure of the title compound. Using the TD-DFT and TD-HF methods, electronic absorption spectra of the title compound have been predicted and a good agreement with the TD-DFT method and the experimental ones is determined. Molecular orbital coefficient analyses reveal that the electronic transitions are mainly assigned to n → π* and π → π* electronic transitions. To investigate the tautomeric stability, optimization calculations at B3LYP/6-31G(d) level were performed for the NH and OH forms of the title compound. Calculated results reveal that the OH form is more stable than NH form. In addition, molecular electrostatic potential and NBO analysis of the title compound were performed at B3LYP/6-31G(d) level of theory.







Similar content being viewed by others
References
Calligaris M, Randaccio L (1987) In: Wilkinson G (ed) Comprehensive coordination chemistry, vol 2. Pergamon, London, pp 715–738
Zhou Y-S, Zhang L-J, Zeng X-R, Vital JJ, You X-Z (2000) J Mol Struct 553:25–30
El-Masry AH, Fahmy HH, Abdelwahed SHA (2000) Molecules 5:1429–1438
Pandey SN, Sriram D, Nath G, De Clercq E (1999) Il Farmaco 54:624–628
Singh WM, Dash BC (1988) Pesticides 22:33–37
Hodnett EM, Dunn WJ (1970) J Med Chem 13:768–770
Desai SB, Desai PB, Desai KR (2001) Heterocycl Commun 7:83–90
Holla BS, Rao BS, Shridhara K, Akberali PM (2000) Il Farmaco 55:338–344
Taggi AE, Hafez AM, Wack H, Young B, Ferraris D, Lectka T (2002) J Am Chem Soc 124:6626–6635
Cohen MD, Schmidt GMJ, Flavian S (1964) J Chem Soc 2041–2051
Hadjoudis E, Vitterakis M, Mavridis IM (1987) Tetrahedron 43:1345–1360
Xu X-X, You X-Z, Sun Z-F, Wang X, Liu H-X (1994) Acta Crystallogr C50:1169–1171
Alarcon SH, Pagani D, Bacigalupo J, Olivieri AC (1999) J Mol Struct 475:233–240
Petek H, Albayrak Ç, Ağar E, Ocak İskeleli N, Şenel I (2007) Acta Cryst E63:o810–o812
Özek A, Albayrak Ç, Odabaşoğlu M, Büyükgüngör O (2007) Acta Cryst C63:o177–o180
Karabıyık H, Ocak İskeleli N, Petek H, Albayrak Ç, Ağar E (2008) J Mol Struct 873:130–136
Koşar B, Büyükgüngör O, Albayrak Ç, Odabaşoğlu M (2004) Acta Cryst C60:o458–o460
Tanak H, Erşahin F, Koysal Y, Ağar E, Işık Ş, Yavuz M (2009) J Mol Mod 15:1281–1290
Petek H, Albayrak Ç, Odabaşoğlu M, Şenel I, Büyükgüngör O (2010) Struct Chem. doi: 10.1007/s11224-010-9598-6
Ogawa K, Harada J (2003) J Mol Struct 647:211
Karabıyık H, Guzel B, Aygün M, Boğa G, Büyükgüngör O (2007) Acta Cryst C63:o215
Ünver H (2001) Spectrosc Lett 34:783
Salman SR, Lindon JC, Farrant RD (1991) Spectrosc Lett 24:1071
Salman SR, Lindon JC, Farrant RD (1993) Magn Reson Chem 31:991
Alarcon SH, Olivieri AC, Nordon A (1995) Tetrahedron 51:4619
Bingöl Alpaslan Y, Tanak H, Ağar E, Erşahin F (2009) Acta Cryst E65:o1842
Tanak H, Yavuz M (2010) J Mol Mod 16:577–587
Bingöl Alpaslan Y, Alpaslan G, Ağar A, Işık Ş (2010) Acta Cryst E66:o510
Trzesowska-Kruszynska A (2010) Struct Chem. doi: 10.1007/s11224-009-9547-4
Cie Stoe (2002) X-AREA (Version 1.18) and X-RED32 (Version 1.04). Stoe & Cie, Darmstadt
Sheldrick GM (1997) SHELXS97 and SHELXL97. University of Göttingen, Göttingen
Burnett MN, Johnson CK (1996) ORTEP 3 Report ORNL-6895. Oak Ridge National Laboratory, Oak Ridge
Schlegel HB (1982) J Comput Chem 3:163
Peng C, Ayala PY, Schlegel HB, Frisch MJ (1996) J Comput Chem 17:49
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Montgomery JA Jr, Vreven T, Kudin KN, Burant JC, Millam JM, Iyengar SS, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson GA, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox JE, Hratchian HP, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Ayala PY, Morokuma K, Voth GA, Salvador P, Dannenberg JJ, Zakrzewski VG, Dapprich S, Daniels AD, Strain MC, Farkas O, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Ortiz JV, Cui Q, Baboul AG, Clifford S, Cioslowski J, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Gonzalez C, Pople JA (2004) Gaussian 03, Revision E.01. Gaussian Inc, Wallingford
Frisch A, Dennington R II, Keith T, Millam J, Nielsen AB, Holder AJ, Hiscocks J (2007) GaussView Reference, Version 4.0. Gaussian Inc, Pittsburgh
Runge E, Gross EKU (1984) Phys Rev Lett 52:997–1000
Stratmann RE, Scuseria GE, Frisch MJ (1998) J Chem Phys 109:8218–8224
Bauernschmitt R, Ahlrichs R (1996) Chem Phys Lett 256:454–464
Casida ME, Jamorski C, Casida KC, Salahub DR (1998) J Chem Phys 108:4439–4449
Miertus S, Scrocco E, Tomasi J (1981) Chem Phys 55:117–129
Barone V, Cossi M (1998) J Phys Chem A 102:1995–2001
Cossi M, Rega N, Scalmani G, Barone V (2003) J Comput Chem 24:669–681
Tomasi J, Mennucci B, Cammi R (2005) Chem Rev 105:2999–3094
Parr RG, Pearson RG (1983) J Am Chem Soc 105:7512
Politzer P, Murray J (2002) Theor Chem Acc 108:134–142
Glendening ED, Badenhoop JK, Reed AE, Carpenter JE, Weinhold F (1995) NBO Version 3.1. Theoretical Chemistry Institute, University of Wisconsin, Madison
Moustakali-Mavridis I, Hadjoudis E, Mavridis A (1978) Acta Cryst B34:3709–3715
Petek H, Albayrak Ç, Odabaşoğlu M, Şenel I, Büyükgüngör O (2008) J Chem Crystallogr 38:901–905
Albayrak Ç, Koşar B, Demir S, Odabaşoğlu M, Büyükgüngör O (2010) J Mol Struct 963:211–218
Allen FH, Kennard O, Watson DG, Brammer L, Orpen AG, Taylor R (1987) J Chem Soc Perkin Trans 2:S1–S19
Petek H, Albayrak Ç, Ocak-İskeleli N, Ağar E, Şenel I (2007) J Chem Crystallogr 37:285–290
Tüfekçi M, Alpaslan G, Macit M, Erdönmez A (2009) Acta Cryst E65:o2143
Yazıcı S, Albayrak Ç, Gümrükçüoglu I, Şenel I, Büyükgüngör O (2010) Acta Cryst E66:o93
Bondi A (1964) J Phys Chem 68:441–450
Bernstein J, Davies RE, Shimoni L, Chang NL (1995) Angew Chem Int Ed Engl 34:1555
Bellamy LJ (1980) The infrared spectra of complex molecules. Chapman and Hall, London vol. 2
Yıldız M, Kılıç Z, Hökelek T (1998) J Mol Struct 441:1–10
Nazır H, Yıldız M, Yılmaz H, Tahir MN, Ülkü D (2000) J Mol Struct 524:241–250
Ünver H, Yıldız M, Zengin DM, Özbey S, Kendi E (2001) J Chem Crystallogr 31:211–216
Salman SR, Kamounah FS (2002) Spectrosc Lett 35:327–335
Özbek N, Kavak G, Özcan Y, İde S, Karacan N (2009) J Mol Struct 919:154–159
Scrocco E, Tomasi J (1978) Adv Quantum Chem 11:115–121
Luque FJ, Lopez JM, Orozco M (2000) Theor Chem Acc 103:343–345
Politzer P, Laurence PR, Jayasuriya K, McKinney J (1985) Environ Health Perspect 61:191–202
Scrocco E, Tomasi J (1973) Topics in current chemistry, vol 7. Springer, Berlin, p 95
Snehalatha M, Ravikumar C, Hubert Joe I, Sekar N, Jayakumar VS (2009) Spectrochim Acta A 72:654–662
Schwenke DW, Truhlar DG (1985) J Chem Phys 82:2418
Gutowski M, Chalasinski G (1993) J Chem Phys 98:4728
Desiraju GR (1991) Acc Chem Res 24:290–296
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Alpaslan, G., Tanak, H., Ağar, A.A. et al. Experimental and computational studies on zwitterionic (E)-2-(1-(2-(4-methylphenylsulfonamido)ethyliminio)ethyl) phenolate. Struct Chem 21, 1027–1036 (2010). https://doi.org/10.1007/s11224-010-9641-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-010-9641-7