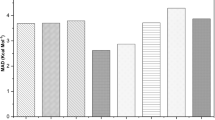
The thermodynamic parameters of lithium, sodium, and potassium single and double sulfate crystals are determined by the method of ab initio calculation of a linear combination of atomic orbitals in the gradient approximation of density functional theory using the software package CRYSTAL09 within the framework of the quasi-harmonic approximation of the Debye theory. It is demonstrated that the standard entropies and heat capacities as well as the temperature dependences are in satisfactory agreement with the available experimental data. The average frequency, Debye temperature, and thermal conductivity coefficient increase with external pressure, whereas the Gruneisen parameter decreases. The dependences of the potentials of free and internal energies on the temperature and volume are expressed through the Birch–Murnaghan equation of state and a square-law dependence on these parameters of their vibrational components. The thermodynamic parameters of lithium-potassium sulfate appear closer to potassium sulfate, whereas for sodium-potassium, they lie between the corresponding parameters for single compounds.
Similar content being viewed by others
References
K. S. Aleksandrov and B. V. Beznosikov, Structural Phase Transitions in Crystals (from the Potassium Sulfate Family) [in Russian], Nauka, Novosibirsk (1993).
I. N. Flerov, A. V. Kartashev, and V. A. Grankina, Fiz. Tverd. Tela, 47, No. 4, 696 (2005).
B. M. Suleiman, M. Gustavsson, E. Karawacki, and A. Lund’en, J. Phys., D30, 2553 (1997).
A. El-Rahman, M. M. El-Desoky, and A. A. El-Sharkawy, J. Phys. Chem. Sol., 60, 119 (1999).
B. M. Suleiman, A. Lunde’n, and E. Karawacki, Solid State Ionics, 136–137, 325 (2000).
M. E. Kassem, S. H. Kandil, E. F. El-Wahidy, and M. El-Gamal, Rev. Phys. Appl., 19, 445 (1984).
L. Abello and C. Pommier, J. Chem. Therm., 17, No. 11, 1023 (1985).
A. G. Nord, Acta Cryst., B32, 982 (1976).
F. McGinnety, Acta Cryst., B28, 2845 (1972).
K. Tanaka, H. Naruse, H. Morikawa, and F. Marumo, Acta Cryst., B47, 581 (1991).
C. B. Pinheiro, M. A. Pimenta, G. Chapuis, and N. L. Speziali, Acta Cryst., B56, 607 (2000).
K. Okada and J. Ossaka, Acta Cryst., B36, 919 (1980).
O. V. Golovko, L. V. Zhuravleva, and Yu. N. Zhuravlev, Russ. Phys. J., 50, No. 1, 101 (2007).
R. Dovesi, V. R. Saunders, C. Roetti, et al., CRYSTAL 06 User’s Manual, University of Torino, Torino (2009).
J. P. Perdew and Y. Wang, Phys. Rev., B45, 13244 (1992).
M. Corno, C. Busco, B. Civalleri, and P. Ugliengo, Phys. Chem. Chem. Phys., 8, 2464 (2006).
B. Civalleri, A. M. Ferrari, M. Llunell, et al., Chem. Mater., 15, 3996 (2003).
F. Birch, J. Geophys. Res., 83, 1257 (1978).
A. M. Molodets, Fiz. Gor. Vzryva, 31, No. 5, 132 (1995).
A. M. Hofmeister, Proc. Nat. Acad. Sci., 104, No. 22, 9192 (2007).
N. de Koker, Earth Planet. Sci. Lett., 292, 392 (2010).
D. Liu, H. M. Lu, J. R. Hardy, and F. G. Ullman, Phys. Rev., B44, No. 14, 7387 (1991).
D. Liu, H. M. Lu, F. G. Ullman, and J. R. Hardy, Phys. Rev., B43, No. 7, 6202 (1991).
J. Mata, X. Solans, M. T. Calvet, et al., J. Phys.–Condens. Matter, 14, 5211 (2002).
R. Murugan, A. Ghule, and H. Chang, J. Phys.–Condens. Matter, 12, 677 (2000).
D. Teeters and R. Frech, Phys. Rev., B26, No. 8, 4132 (1982).
F. E. Bernardin and W. S. Hammack, Phys. Rev., B54, No. 10, 7026 (1996).
Z. Wu, E. Zhao, H. Xiang, et al., Phys. Rev., B76, No. 054115 (2007).
Internet resource: http://www.update.uu.se/~jolkkonen/pdf/CRC_TD.pdf.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Izvestiya Vysshikh Uchebnykh Zavedenii, Fizika, No. 6, pp. 31–38, June, 2013.
Rights and permissions
About this article
Cite this article
Zhuravlev, Y.N., Bugaeva, I.A. & Zhuravleva, L.V. Ab Initio Study of Thermodynamic Properties of Lithium, Sodium, and Potassium Sulfates. Russ Phys J 56, 638–646 (2013). https://doi.org/10.1007/s11182-013-0079-4
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11182-013-0079-4