Abstract
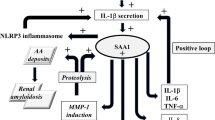
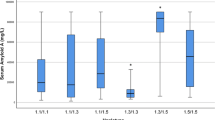
The Amyloid A1 (AA1) and A2 (AA2) proteins, which result from proteolytic cleavage of the Serum Amyloid A1 (SAA1) and A2 (SAA2) proteins, are major protein components of the Amyloid A deposits found in secondary amyloidosis. This study determines frequency of serum amyloid A2 alleles (α, β) in healthy Turkish, Azerbaijani, and Kazakh subjects. Two hundred Turkish, sixty-five Azerbaijani and sixty-five Kazakh healthy individuals were studied by previously described the PCR-RFLP methods. Our data revealed that the frequencies of the α and β alleles at the SAA2 locus in the Turkish healthy population were different when compared to those in Azerbaijani and Kazakh healthy populations (P = 0.014 and 0.02), respectively. In contrast, the difference between α and β alleles at the SAA2 locus was not different in both Kazakh and Azerbaijani healthy populations (P = 0.882).
Similar content being viewed by others
REFERENCES
Parpelee, D.C., Titani, K., Ericson, L.H., et al., Amino Acid Sequence of Amyloid-Related Apoprotein (Apo SAA1) from Human High-Density Lipoprotein, Biochemistry, 1982, vol. 21, pp. 3298–3303.
Dwulet, F.E., Wallace, D.K., and Beouson, M.D., Amino Acid Structures of Multiple Forms of Amyloid-Related Serum Protein SAA from a Single Individual, Biochemistry, 1988, vol. 27, pp. 1677–1682.
Ramadori, G., Sipe, J.D., Dinarello, C.A., et al., Pretranslational Modulation of Acute-Phase Hepatic Protein Synthesis by Murine Recombinant Interleukin 1 (IL-1) and Purified Human IL-1, J. Exp. Med., 1985, vol. 162, pp. 930–942.
Kisilevsky, R., Serum Amyloid A (SAA); a Protein without a Function: Some Suggestions with Reference to Cholesterol Metabolism, Med. Hypothes., 1991, vol. 35, pp. 337–341.
Zimlichman, S., Danon, A., Hathan, I., et al., Serum Amyloid A, an Acute Phase Protein Inhibits Platelet Activation, J. Lab. Clin. Med., 1990, vol. 116, pp. 180–186.
Husby, G., Marhaug, G., Dowton, B., et al., Serum Amyloid A (SAA): Biochemistry, Genetics and the Pathogenesis of AA Amyloidosis, Amyloid: Int. J. Exp. Clin. Invest., 1994, vol. 1, pp. 119–137.
Sipe, J.D., Serum Amyloid A Protein Classification: A Preliminary Report of a Subcommittee of the International Society of Amyloidosis, Amyloid: Int. Exp. Clin. Invest., 1995, vol. 2, pp. 69–70.
Yamada, T., Okuda, Y., and Itoh, Y., The Frequency of Serum Amyloid A2 Alleles in the Japanese Population, Amyloid: Int. Exp. Clin. Invest., 1998, vol. 5, pp. 208–211.
Hazenberg, B.P.C., Limburg, P.C., Bijzet, J., and Van Rijswijk, M.H., SAA Isoform Patterns in Patients with and without AA Amyloidosis, Amyloid and Amyloidosis, Kisilevsky, R., Benson, M.D., Frangione, B., et al., Eds., New York: Parthenon, 1993, pp. 90–92.
Kluve-Beckerman, B., Drumm, M.L., and Benson, M.D., None-Expression of Serum Amyloid A Three (SAA3) Gene, DNA Cell Biol., 1991, vol. 10, pp. 651–661.
Liepnieks, J.J., Kluve-Beckerman, B., and Benson, M.D., Characterization of Amyloid A Protein in Human Secondary Amyloidosis, the Predominant Deposition of Serum Amyloid A1, Biochim. Biophys. Acta, 1995, vol. 1270, pp. 81–86.
Moriguchi, M., Terai, C., Koseki, Y., et al., Influences of Genotypes at SAA1 and SAA2 Loci on the Development and the Length of Latent Period of Secondary AA Amyloidosis in Patients with Rheumatoid Arthritis, Hum. Genet., 1999, vol. 105, pp. 360–366.
Madisen, L., Hoar, D.I., Holrayd, C.D., et al., DNA Banking: The Effects of Storage of Blood and Isolated DNA on the Integrity of DNA, Am. J. Med. Genet., 1987, vol. 27, pp. 379–390.
Faulkes, D.J., Betts, J.C., and Woop, P., Characterization at Five Human Serum Amyloid A1 Alleles, Amyloid: Int. J. Exp. Clin. Invest., 1994, vol. 1, pp. 255–262.
Cazeneuve, C., Ajrapetyan, H., Papin, S., et al., Identification of MEFV-Independent Modifying Genetic Factors for Familial Mediterranean Fever, Am. J. Hum. Genet., 2000, vol. 67, pp. 1136–1143.
Gershoni-Baruch, R., Brik, R., Zacks, N., et al., The Contribution of Genotypes at the MEFV and SAA1 Loci to Amyloidosis and Disease Severity in Patients with Familial Mediterranean Fever, Arthritis Rheum., 2003, vol. 48, pp. 1148–1155.
Author information
Authors and Affiliations
Additional information
From Genetika, Vol. 41, No. 7, 2005, pp. 986–989.
Original English Text Copyright © 2005 by Hakki Tastan, Ozlem Osmanagaoglu, Ayla Tuzun.
The text was submitted by the authors in English.
Rights and permissions
About this article
Cite this article
Tastan, H., Osmanagaoglu, O. & Tuzun, A. The Frequencies of the Serum Amyloid A2 Alleles in Healthy (Turkish, Azerbaijani, and Kazakh) Populations. Russ J Genet 41, 805–807 (2005). https://doi.org/10.1007/s11177-005-0164-z
Received:
Issue Date:
DOI: https://doi.org/10.1007/s11177-005-0164-z