Abstract
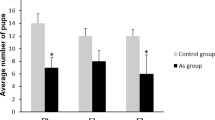
We examined the effects of nickel sulfate at doses 0.5 to 5.0 mg/kg (1/200–1/20 LD50) on the frequency of dominant lethal mutations and double-strand DNA breaks (DSBs) in germline cells and on an increase in frequency in gene mutations W y in pigment cells of first-generation mice. The results indicated that spermatogenesis stages most sensitive to nickel sulfate (at a dose of 1.0 mg/kg) are spermatozoids, early spermatids, late spermatocytes, and stem spermatogonia. No statistically significant increase in the total TSB level was detected in spermatozoids 4 weeks after exposure. At the same time, a significant (P < 0.05) increase in percentage of cells with an extremely high level of DNA fragmentation (supposedly apoptotic cells) was observed upon exposure at a dose of 0.5 mg/kg. Nickel sulfate at doses of 5.0 and 1.0 mg/kg induced a marked increase in the c-kit gene expression in pigment cells of heterozygous first-generation WR mice as compared to control (P < 0.001). It was shown that the nonobservable adverse effect level (NOAEL) of nickel sulfate on the dominant lethal mutation frequency and gene mutations was 1/200 LD50, while the lowest observable adverse effect level (LOAEL) was 1/100 LD50.
Similar content being viewed by others
REFERENCES
Chashin, V.P., Artunina, G.P., and Nortseth, T., Congenital Defects, Abortion and Other Health Effects in Nickel Refinery Workers, Sci. Total Environ., 1994, vol. 148, pp. 257–291.
Toxicological Profile for Nickel (Update), US Department of Health & Human Services Public Health Service, Agency for Toxic Substances and Diases Registry, 1997, pp. 51–52, 82, 124–129.
Klein, C.B., Kargasin, B., and Su, L., Metal Mutagenic Chinese Hamster Cell Lines, Environ. Health Perspect., 1994, vol. 102,suppl. 3, pp. 63–67.
Miki, H., Kasprzak, K.S., Kenney, S., and Heine, U.I., Inhibition of Intercellular Communication by Nickel (II): Antagonistic Effect of Magnesium, Carcinogenesis, 1987, vol. 8, pp. 1757–1760.
Sobti, R.C. and Gill, R.K., Incidence of Micronuclei and Abnormalities in the Head of Spermatozoa Caused by Salts of a Heavy Metal Nickel, Cytologia, 1988, vol. 54, pp. 249–254.
Dhir, H., Agarwal, K., Sharma, A., et al., Modifying Role of Phyllanthes emlica and Ascorbic Acid against Nickel Clastogenicity in Mice, Cancer Lett., 1991, vol. 59, pp. 9–18.
Andersen, O., Effects of Coal Combustion Products and Metal Compounds on Sister Chromatid Exchange (SCE) in a Macrophage Cell Line, Environ. Health Perspect., 1983, vol. 47, pp. 239–253.
Arrouijal, F.Z., Marsin, D., Hildebrand, H.F., et al., Differences in Genotoxic Activity of α-Ni3S2 on Human Lymphocytes from Nickel-Hypersentized and Nickel-Unsensitized Donors, Mutagenesis, 1992, vol. 7, no.3, pp. 183–187.
Saxholm, H.J.K., Reith, A., and Brogger, A., Oncogenic Transformation and Cell Increased Sister Chromatid Exchange in Human Lymphocytes by Nickel Subsulfide, Cancer Res., 1981, vol. 41, pp. 4136–4139.
Wulf, H.C., Sister Chromatid Exchanges in Human Lymphocytes Exposed to Nickel and Lead, Dan. Med. Bull., 1980, vol. 27, pp. 40–42.
Sahu, R.K., Katsifis, S.P., Kinney, P.L., and Christie, N.T., Effect of Nickel Sulfate, Lead Sulfate, and Sodium Arsenite Alone and with UV Light on Cultured Human Lymphocytes, J. Mol. Toxicol., 1989, vol. 2, pp. 129–136.
Deknudt, G.H. and Leonard, A., Mutagenicity Tests with Nickel Salts in the Male Mouse, Toxicology, 1982, vol. 25, pp. 289–292.
Jacquet, P. and Mayence, A., Application of the In Vitro Embryo Culture to the Study of the Mutagenic Effects of Nickel in Male Germ Cells, Toxicol. Lett., 1982, vol. 11, pp. 193–197.
Patierno, S.R., Sugiyama, M., Basilion, J.P., and Costa, M., Preferential DNA-Protein Crosslinking by NiCl2 in Magnesium-Insoluble Regions of Fractionated Chinese Hamster Ovary Cell Chromatin, Cancer. Res., 1985, vol. 45, pp. 5787–5794.
Kasprzak, K.S., The Role of Oxidative Damage in Metal Carcinogenicity, Chem. Res. Toxicol., 1991, vol. 4, pp. 604–615.
Hartwig, A., Mullenders, L.H.F., Schlepegrell, R., et al., Nickel(II) Interferes with the Incision Step in Nucleotide Excision Repair in Mammalian Cells, Cancer. Res., 1994, vol. 54, pp. 4045–4051.
Lei, Y.X., Chen, J.K., and Wu, Z.L., Detection of DNA Strand Breaks, DNA-Protein Crosslinks, and Telomerase Activity in Nickel-Transformed BALB/c 3T3 Cells, Teratogen. Carcinogen. Mutagen., 2001, vol. 21, no.6, pp. 463–471.
Elakov, A.L., Mironova, J.V., Osipov, A.N., and Sypin, V.D., Comparative Study of Nickel and Cadmium Influence on Spleen Lymphocytes of Different Age Mice, Environment and Human Health: The Complete Works of International Ecologic Forum, June 29–July 2, 2003, St. Petersburg, Russia, Sofronov, G.A., Ed., St. Petersburg: SpecLit, 2003, p. 577.
Mastromatteo, E., Jant Memorial Lecture: Nickel, Am. Ind. Hyg. Assoc. J., 1986, vol. 47, pp. 589–601.
Blandova, Z.K., Dushkin, V.A., Malashenko, A.M., et al., Linii laboratornykh myshei dlya mediko-biologicheskikh issledovanii (Laboratory Strains of Mice for Medical-Biological Investigations), Moscow: Nauka, 1983, 199 p.
Malashenko, A.M. and Surkova, N.I., WR, A New Mouse Line Highly Sensitive to the Cytogenetic Effect of ThioTEP, Tsitol. Genet., 1979, vol. 13, pp. 387–391.
Yang-Feng, T.L., Ulrich, A., and Franke, A., The Oncogene c-kit (KIT) Is Located on Man Chromosome 4 and Mouse Chromosome 5, Cytogenet. Cell Genet., 1987, vol. 46, p. 723.
Shevchenko, V.A and Pomerantseva, M.D., Dominant Lethal Mutations, in Geneticheskie posledstviya ioniziruyushchikh izluchenii (Genetic Consequences of Ionizing Radiation), Moscow: Nauka, 1985, pp. 41–42.
Ehling, U.H., Machemer, L., Buselmaier, W., et al., Standard Protocol for the Dominant Lethal Test on Male Mice, Arch. Toxicol., 1978, vol. 39, pp. 173–185.
Fahrig, R., A Mammalian Spot-Test: Induction of Genetic Alterations in Pigment Cells of Mouse Embryos with X-Rays and Chemical Mutagens, Mol. Gen. Genet., 1975, vol. 138, no.4, pp. 309–314.
Singh, N.P. and Stephens, R.E., X-Ray-Induced DNA Double-Strand Breaks in Human Sperm, Mutagenesis, 1998, vol. 13, no.1, pp. 75–79.
Collins, A.R., Ma, A.G., and Duthie, S.J., The Kinetics of Repair of Oxidative DNA Damage (Strand Breaks and Oxidized Pyrimidine Dimers in Human Cells), Mutat. Res., 1995, vol. 336, no.1, pp. 69–77.
Domshlak, M.G., Chirkova, E.M., and Strekalova, E.E., Mutagenic Effect of Ethylenimine (EI) on Mouse Gametes upon Inhalation or Introduction into the Stomach, Laboratornye zhivotnye v meditsinskikh issledovaniyakh (Laboratory Animals in Medical Studies, Moscow: Nauch.-Issled. Lab. Eksp. Biol. Modelei, 1974, pp. 42–44.
Domshlak, M.G., Genetic Effect of Low Doses of Industrial Chemicals, in Meditsina truda na predpriyatiyakh g. Moskvy (Occupational Medicine at Moscow Enterprises), Moscow: Inst. Med. Truda, 1998, pp. 148–150.
Fomenko, V.N., Domchlak, M.G., Katosova, L.D., et al., Mutagenic and Gonadotropic Effects of Styrene, Proc. of the Finish-Soviet Joint Symp. on Industrial Hygiene and Toxicology, Helsinki, 1984, pp. 48–57.
Fomenko, V.N., Domchlak, M.G., Katosova, L.D., et al., General Toxic and Specific Chrome Effect, Proc. 6th Finish-Soviet Joint Symp. on Occupational Health, Rovaniemi, 1987, pp. 26–31.
Genroso, W.M., Russel, L.B., Huff, S.W., et al., Effect of Dose on the Induction of Dominant Lethal Mutations and Heritable Translocations with Methyl Methanesulfonate in Male Mice, Genetics, 1974, vol. 77, pp. 741–752.
Bateman, A.J., Mutagenic Sensitivity of Maturing Germ Cells in the Male Mouse, Heredity, 1958, vol. 12, pp. 213–232.
Ehling, U.H., Differential Spermatogenic Response of Mice to the Induction of Mutations by Antineoplastic Drugs, Iutat. Res., 1974, vol. 26, pp. 285–295.
Ehling, U.H. and Neuhauser-Klaus, A., Induction of Specific-Locus and Dominant Lethal Mutations in Male Mice by in N-Propyl and Isopropyl Methanesulfonate, Mutat. Res., 1995, vol. 328, pp. 73–82.
Burlakova, E.B., Distant Consequences of the Integral Effect of Low Doses and Damaging Environmental Factors on Population Viability, in Budushchee natsii (Future of the Nation), Moscow, 1995, part 2, pp. 64–76.
Svendsgaard, D. and Calabrase, E.J., U-Shaped Dose Response Relationships in Toxicology, XXI Medichen Congr., 18–21 October, 1994, Melbourne, 1994, pp. 106–124.
Sakkas, D., Mariethoz, E., Manicardi, G., et al., Origin of DNA Damage in Ejaculated Human Spermatozoa, Rev. Reprod., 1999, vol. 4, pp. 31–37.
McPherson, S.M.G. and Longo, F.J., Localization of DNase I-Hypersensitive Regions during Rat Spermatogenesis: Stage-Dependent Patterns and Unique Sensitivity of Elongating Spermatids, Mol. Reprod. Dev., 1992, vol. 31, pp. 268–279.
McPherson, S.M.G. and Longo, F.J., Nicking of Rat Spermatid and Spermatozoa DNA: Possible Involvement of DNA Topoisomerase II, Dev. Biol., 1993, vol. 158, pp. 122–130.
McPherson, S.M.G. and Longo, F.J., Chromatin Structure-Function Alterations during Mammalian Spermatogenesis: DNA Nicking and Repair in Elongating Spermatids, Eur. J. Histochem., 1993, vol. 37, pp. 109–128.
Sakkas, D., Manicardi, G.C., Bianchi, P.G., et al., Relationship between the Presence of Endogenous Nicks and Sperm Chromatin Packaging in Maturing and Fertilizing Mouse Spermatozoa, Biol. Reprod., 1995, vol. 52, pp. 1149–1155.
Suda, T., Takahashi, T., Golstein, P., and Nagata, S., Molecular Cloning and Expression of the Fas Ligand, a Novel Member of the Tumor Necrosis Factor Family, Cell (Cambridge, Mass.), 1993, vol. 75, pp. 1169–1178.
Lee, J., Richburg, J.H., Younkin, S.C., and Boekelheide, K., The Fas System Is a Key Regulator of Germ Cell Apoptosis in the Testis, Endocrinology, 1997, vol. 138, pp. 2081–2088.
Domshlak, M.G., Quantitation of Genetic Risk and Relative Genetic Efficiency for Some Alkylating Agents, Zh. Med. Trula Prom. Ekol., 2003, no. 9, pp. 33–39.
Domshlak, M.G., Chirkova, E.M., Malashenko, A.M., et al., Registration of Changes in the W y Gene in Somatic Mouse Cells As a Means to Estimate the Genetic Effect in Toxicological Studies, Gig. Tr. Prof. Zabol., 1981, no. 7, pp. 52–53.
Shustov, V.Ya., Mikroelementy v gematologii (Microelements in Hematology), Moscow: Meditsina, 1967.
Jasim, S. and Tjalve, H., Effect of Sodium Pyridimethione on the Uptake and Distribution of Nickel, Cadmium and Zinc in Pregnant and Non-Pregnant, Toxicology, 1986, vol. 38, pp. 327–350.
Leonard, A., Gerber, G.B., and Jacouet, P., Carcinogenicity, Mutagenicity and Teratogenisity of Nickel, Mutat. Res., 1981, vol. 87, pp. 1–15.
Ehling, U.H., Evaluation of Genetic Hazards in Man from Radiation and Chemical Mutagens, Radiobiological Equivalents of Chemical Pollutants, International Atomic Energy, 1980, pp. 71–81.
Author information
Authors and Affiliations
Additional information
__________
Translated from Genetika, Vol. 41, No. 7, 2005, pp. 894–901.
Original Russian Text Copyright © 2005 by Domshlak, Elakov, Osipov.
Rights and permissions
About this article
Cite this article
Domshlak, M.G., Elakov, A.L. & Osipov, A.N. Genetic Effects Induced by Nickel Sulfate in Germline and Somatic Cells of WR Mice. Russ J Genet 41, 728–734 (2005). https://doi.org/10.1007/s11177-005-0152-3
Received:
Issue Date:
DOI: https://doi.org/10.1007/s11177-005-0152-3