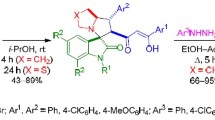
Abstract
Cyclocondensation of cyclohexene-4-carbaldehyde in the presence of morpholine with CH acids [malonodinitrile, dimedone, 1,3-cyclohexanedione, ethyl acetoacetate, cyanothioacetamide, β-aminophenol, resorcinol, and 4-(cyclopent-1-enyl)morpholine] yields the corresponding 4-(cyclohex-3-enyl)-substituted 4H-chromenes, 4H-thiopyrans, 1,4,5,6,7,8-hexahydroquinolines, 1,4-dihydropyridines, and 6,7-dihydro-5H-[1]pyrindines.
Similar content being viewed by others
REFERENCES
Tietze, L.F., Chem. Rev., 1996, vol. 96, no.1, p. 115.
Litvinov, V.P., Usp. Khim., 2003, vol. 72, no.1, p. 75.
Dyachenko, V.D., Doctoral (Chem.) Dissertation, Moscow, 1998.
Gorobets, E.V., Miftakhov, M.S., and Valeev, F.A., Usp. Khim., 2000, vol. 69, no.12, p. 1091.
Ugi, I., Abstracts of Papers, III Vserossiiskii simpozium po organicheskoi khimii “Strategiya i taktika organicheskogo sinteza” (III Russian Symp. “Strategy and Tactics of Organic Synthesis”), Yaroslavl, 2001, p. 2.
Smith, W.A., Bochkov, A.F., and Caple, R., Organic Synthesis: The Science behind the Art, London: Roy. Soc. Chem., 1998.
Dyachenko, V.D., in Novye dostizheniya v khimii karbonil'nykh soedinenii: Sbornik nauchnykh trudov (New Achievements in Chemistry of Carbonyl Compounds: Coll. of Scientific Papers), Saratov, 2000, p. 60.
Dyachenko, A.D., Desenko, S.M., and Dyachenko, V.D., Khim. Geterotsikl. Soedin., 2002, no. 6, p. 845.
Dyachenko, V.D., Nesterov, V.N., Krivokolysko, S.G., and Litvinov, V.P., Izv. Ross. Akad. Nauk, Ser. Khim., 1997, no. 1, p. 196.
Dyachenko, V.D., Krivokolysko, S.G., and Litvinov, V.P., Khim. Geterotsikl. Soedin., 1998, no. 1, p. 81.
Dyachenko, V.D., Nesterov, V.N., Krivokolysko, S.G., and Litvinov, V.P., Khim. Geterotsikl. Soedin., 1997, no. 6, p. 785.
Comprehensive Organic Chemistry. The Synthesis and Reactions of Organic Compounds, Barton, D. and Ollis, W.D., Eds., Oxford: Pergamon, 1979.
Drabkina, A.A. and Tsizin, Yu.S., Zh. Obshch. Khim., 1974, vol. 44, no.2, p. 450.
Stork, G. and Landesman, H.K., J. Am. Chem. Soc., 1956, vol. 78, no.19, p. 5128.
Litvinov, V.P., Promonenkov, V.K., Sharanin, Yu.A., and Shestopalov, A.M., Itogi Nauki Tekh., Ser.: Org. Khim., 1989, vol. 17, p. 72.
Litvinov, V.P., Rodinovskaya, L.A., Sharanin, Yu.A., Schestopalov, A.M., and Senning, A., Sulfur Rep., 1992, vol. 13, no.1, p. 1.
Kaigorodova, E.A., Vasilin, V.K., and Krapivin, G.D., Available from VINITI, Moscow, 2001, no. 1901-V; Ref. Zh. Khim., 2002, 02.04-19Zh213Dep.
Issac, Y.A., Ali, M.S., and Erian, A.W., Sci. Pharm., 2000, vol. 68, no.4, p. 389.
El-Abadehan, M.M., Sabri, S.S., Al-Ashqar, A., Mion, P., Bompart, J., and Calas, M., Phosphorus, Sulfur, Silicon, Relat. Elem., 1998, vols. 134–135, no.1, p. 21.
Attia, A., Abo-Ghalia, M.H., and El-Salem, O.I.A., Pharmazie, 1995, vol. 50, no.7, p. 455.
Hussein, A.M., Abu-Shanal, F.A., and Ishak, E.A., Phosphorus, Sulfur, Silicon, Relat. Elem., 2000, vol. 159, no.1, p. 55.
Mohareb, R.M., Hoda, H.Z., Elkhoyly, Y.M., and Rasha, R.A., Phosphorus, Sulfur, Silicon, Relat. Elem., 1999, vol. 155, no.1, p. 215.
US Patent 6 329 388, 2000, Ref. Zh. Khim., 2002, 02.20-19O73P.
US Patent 5 656 638, 1997, Chem. Abstr., 1997, vol. 127, no. 16, 220 647x.
GDR Patent 275 688, 1990, Ref. Zh. Khim., 1990, 22O60P.
Sharanin, Yu.A., Shestopalov, A.M., Nesterov, V.N., Melenchuk, S.N., Promonenkov, V.K., Shklover, V.E., Struchkov, Yu.T., and Litvinov, V.P., Zh. Org. Khim., 1989, vol. 25, no.6, p. 1323.
Dyachenko, V.D., Krivokolysko, S.G., Sharanin, Yu.A., and Litvinov, V.P., Zh. Org. Khim., 1997, vol. 33, no.7, p. 1084.
Dyachenko, V.D. and Litvinov, V.P., Khim. Geterotsikl. Soedin., 1997, no. 7, p. 995.
Dyachenko, V.D. and Litvinov, V.P., Zh. Org. Khim., 1998, vol. 34, no.4, p. 592.
Nakanishi, K., Infrared Absorption Spectroscopy, Tokyo: Nankido, 1962.
Sharanin, Yu.A., Shestopalov, A.M., Litvinov, V.P., Klokol, G.V., Mortikov, V.Yu., and Demerkov, A.S., Zh. Org. Khim., 1988, vol. 24, no.4, p. 854.
RF Patent 2 083 572, 1997, Ref. Zh. Khim., 1998, 6O50P.
US Patent 5 514 706, 1996, Ref. Zh. Khim., 1998, 13O40P.
Kartsev, V.G., in Izbrannye metody sinteza i modifikatsii geterotsiklov (Selected Methods for Synthesis and Modification of Heterocycles), Moscow: IBS, 2003, p. 534.
Author information
Authors and Affiliations
Additional information
__________
Translated from Zhurnal Obshchei Khimii, Vol. 75, No. 10, 2005, pp. 1612–1619.
Original Russian Text Copyright © 2005 by Dyachenko.
Rights and permissions
About this article
Cite this article
Dyachenko, V.D. Cyclohexene-4-carbaldehyde in the Synthesis of 4-(Cyclohex-3-enyl)-Substituted 4H-Chromenes, 4H-Thiopyrans, 1,4,5,6,7,8-Hexahydroquinolines, 1,4-Dihydropyridines, Pyridines, and 6,7-Dihydro-5H-[1]pyrindines. Russ J Gen Chem 75, 1537–1544 (2005). https://doi.org/10.1007/s11176-005-0463-z
Received:
Issue Date:
DOI: https://doi.org/10.1007/s11176-005-0463-z