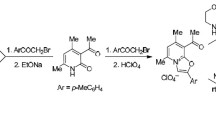
Abstract
When heated in polyphosphoric acid, the products of amidophenacylation of the available triphenylphosphoranylideneacetonitrile undergo the Robinson-Gabriel cyclization followed by other transformations. Treatment of the resulting mixture with sodium perchlorate gave quaternary phosphonium salts of two types, viz. (2, 5-diaryl-1, 3-oxazol-4-ylmethyl) triphenylphosphonium perchlorates and [2-aryl-5-hydroxynaphtho[2, 1-d][1,3]oxazol-4-yl]triphenylphosphonium perchlorates. The first of these products was identified by alkaline dephosphorylation, and the structure of one of the representatives of the second class of compounds was established by X-ray diffraction.
Similar content being viewed by others
REFERENCES
Drach, B.S., Brovarets, V.S., and Smolii, O.B., Zh. Obshch. Khim., 2002, vol. 11, p. 1764.
Drach, B.S., Brovarets, V.S., and Smolii, O.B., Abstract of Papers, Materialy 1 mezhdunarodnoi konferentsii-Khimiya i biologicheskaya aktivnost’ azotistykh geterotsiklov i alkaloidov-(Proc. 1st. Int. Conf. “Chemistry and Biological Activity of Nitrogenous Heterocycles and Alkaloids”), Moscow, 2001, vol. 1, p. 69.
Smolii, O.B., Panchishin, S.Ya., Romanenko, E.A., and Drach, B.S., Zh. Obshch. Khim., 1995, vol. 65, no.4, p. 583.
Van Meervelt, L., Smolii, O.B., Mishchenko, N.I., Shakhnin, D.B., Romanenko, E.A., and Drach, B.S., Tetrahedron, 1996, vol. 52, no.26, p. 8835.
Smolii, O.B., Panchishin, S.Ya., Budnik, L.V., Romanenko, E.A., and Drach, B.S., Zh. Obshch. Khim., 1997, vol. 67, no.3, p. 391.
Smolii, O.B., Panchishin, S,Ya., Van Meervelt, L., Mishchenko, N.I., Romanenko, E. A., and Drach, B.S., Zh. Obshch. Khim., 1998, vol. 68, no.4, p. 585.
Smolii, O.B., Panchishin, S.Ya., Romanenko, E.A., and Drach, B.S., Zh. Obshch. Khim., 1999, vol. 69, no.10, p. 1652.
Smolii, O.B., Panchishin, S.Ya., Pirozhenko, V.V., and Drach, B.S., Zh. Obshch. Khim., 2001, vol. 71, no.11, p. 1830.
Heterocyclic Compounds, Elderfield, R.C., Ed., New York: Wiley, 1957, vol. 5. Translated under the title Geterotsiklicheskie soedineniya, Moscow: Inostrannaya Literatura, 1961, vol. 5, p. 243.
Lister, J. and Robinson, R., J. Chem. Soc. 1912, vol. 101, p. 1297.
Naumov, V.A. and Vilkov, L. V., Molekulyarnye struktury fosfororganicheskikh soedinenii (Molecular Structures of Organophosphorus Compounds), Moscow: Nauka, 1986.
Allen, F. H., Kennard, O., Watson, D. G., Brammer, L., Orpen, A. G., and Tailor, R., J. Chem. Soc., Perkin Trans. 2, 1987, no. 12, p. S1.
Sheldric, G.M., SHELXS-86. Program for the Solution of Crystal Structures, Goettingen: Univ. of Goettingen, 1986.
Sheldric, G.M., SHELXS-93. Program for the Refinement of Crystal Structures, Goettingen: Univ. of Goettingen, 1993.
Author information
Authors and Affiliations
Additional information
__________
Translated from Zhurnal Obshchei Khimii, Vol. 75, No. 4, 2005, pp. 556–560.
Original Russian Text Copyright © 2005 by Panchishin, Smolii, Chernega, Rusanov, Drach.
Rights and permissions
About this article
Cite this article
Panchishin, S.Y., Smolii, O.B., Chernega, A.N. et al. Cyclocondensations of Amidophenacylation Products of Triphenylphosphoranylideneacetonitrile. Russ J Gen Chem 75, 518–522 (2005). https://doi.org/10.1007/s11176-005-0264-4
Received:
Issue Date:
DOI: https://doi.org/10.1007/s11176-005-0264-4