Abstract
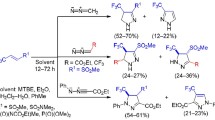
Ethyl 2- and 5-cyclopropylfuran-3-carboxylates react with the system comprising Paraform, hydrogen chloride, and zinc chloride to form chloromethyl derivatives. This process is accompanied by selective cleavage of the cyclopropane ring, leading to appearance of a γ-chloropropyl fragment. Phosphorylation of the resulting halides with sodium diethyl phosphite in benzene involves exclusively the chloromethyl group.
Similar content being viewed by others
REFERENCES
Parker, K.A. and Johnson, N.S., Tetrahedron Lett., 1968, no. 17, pp.1329–1332.
Epshtein, A.E., Izv Akad. Nauk SSSR, Ser. Khim., 1978, no 2, pp. 500–503.
Author information
Authors and Affiliations
Additional information
Translated from Zhurnal Obshchei Khimii, Vol. 74, No. 8, 2004, pp. 1279–1281.
Original Russian Text Copyright © 2004 by Pevzner.
Rights and permissions
About this article
Cite this article
Pevzner, L.M. Reactions of esters of cyclopropylfurancarboxylic acids in the system paraform-hydrogen chloride-zinc chloride and phosphorylation of reaction products. Russ J Gen Chem 74, 1182–1184 (2004). https://doi.org/10.1007/s11176-005-0134-0
Received:
Issue Date:
DOI: https://doi.org/10.1007/s11176-005-0134-0