Abstract
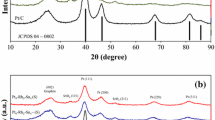
Structural characteristics and electrocatalytic activity in the methanol oxidation reaction in an alkaline solution of the Raney nickel promoted by a platinum-ruthenium mixture are studied with the aid of methods of scanning electron microscopy, x-ray diffraction microanalysis, BET, and measurements of cyclic voltamograms and polarization curves. Distributions of all components of the system under investigation (Al, Ni, Pt, Ru, O) at the surface of the catalyst, the average size of whose particles amounts to 20–30 µm, are established. It is shown that a number of parameters (composition and quantity of the promoting mixture, temperature and concentration of methanol and alkali, amount of the active mass of the electrode) exert an influence on the methanol oxidation rate. The catalyst on the basis of the Raney nickel promoted by 10 wt % Pt/Ru (1/9 at. %) exhibits maximum activity in the methanol electrooxidation reaction in a solution that contains 4 M CH3OH in 6 M KOH. Upon elevating temperature by 20°C in the temperature interval 40 to 80°C the reaction accelerates by 2–3 times.
Similar content being viewed by others
REFERENCES
Vielstich, W., Brenstoffelementen, Weinheim: Chemie, 1965.
Korovin, N.V., Elektrokhimicheskie generatory (Electrochemical Generators), Moscow: Energiya, 1974.
Vitvitskaya, G.V. and Daniel'-Bek, V.S., Elektrokhimiya, 1965, vol. 1, p. 759.
Daniel'-Bek, V.S. and Vitvitskaya, G.V., Elektrokhimiya, 1966, vol. 2, p. 303.
Parsons, R. and Vander Noot, T., J. Electroanal. Chem., 1988, vol. 257, p. 9.
Lamy, C., Leger, J.-M., and Srinivasan, S., Modern Aspects of Electrochemistry, Bokris, J.O'M., Conway, B.E., and White, R.E., Eds., New York: Plenum, 2001, vol. 34, p. 53.
Xia, X.H., Iwasita, T., Ge, F., and Vielstich, W., Electrochim. Acta, 1996, vol. 41, p. 711.
Wasmus, S. and Kuver, A., J. Electroanal. Chem., 1999, vol. 461, p. 14.
Leger, J.-M., J. Appl. Electrochem., 2001, vol. 31, p. 767.
Wang, H., Wingender, C., Baltruschat, H., Lopez, M., and Rutz, M.T., J. Electroanal. Chem., 2001, vol. 509, p. 163.
Morimoto, I. and Yeager, E.B., J. Electroanal. Chem., 1998, vol. 444, p. 95.
Biswas, P.S., Nodasaka, Y., and Enyo, M., J. Appl. Electrochem., 1996, vol. 26, p. 30.
Tripkovic, A.V., Popovic, K.Dj., Momcilovic, J.D., and Drazic, D.M., J. Electroanal. Chem., 1996, vol. 418, p. 9.
Morallon, E., Rodes, A., Vazques, J.L., and Perez, J.M., J. Electroanal. Chem., 1995, vol. 391, p. 149.
Chen, S. and Schell, M., J. Electroanal. Chem., 1999, vol. 478, p. 108.
Abracham, K.M. and Jiang, Z., J. Electrochem. Soc., 1996, vol. 143, p. 1.
Jenseit, W., Khalil, A., and Wendet, H., J. Appl. Electrochem., 1990, vol. 20, p. 893.
Takasu, Y., Fujiwara, T., and Murakami, Y., J. Electrochem. Soc., 2000, vol. 147, p. 4421.
Abramzon, O.S., Chernyshov, S.F., and Pshenichnikov, A.G., Elektrokhimiya, 1976, vol. 12, p. 1667.
Pshenichnikov, A.G., Kudryavtseva, Z.I., and Burkal'tseva, L.A., Elektrokhimiya, 1980, vol. 16, p. 151.
Antolini, E., Giorgi, L., Cardelline, F., and Passalacqua, E., J. Solid State Lett., 2001, vol. 5, p. 131.
Author information
Authors and Affiliations
Corresponding author
Additional information
__________
Translated from Elektrokhimiya, Vol. 41, No. 12, 2005, pp. 1422–1430.
Original Russian Text Copyright © 2005 by Karichev, Tarasevich, Efremov, Bogdanovskaya, Kapustin.
Rights and permissions
About this article
Cite this article
Karichev, Z.R., Tarasevich, M.R., Efremov, B.N. et al. Structural Characteristics of the Raney Nickel Promoted by a Platinum-Ruthenium Mixture and Its Electrocatalytic Activity in the Methanol Oxidation Reaction in Alkaline Media. Russ J Electrochem 41, 1265–1273 (2005). https://doi.org/10.1007/s11175-005-0214-9
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s11175-005-0214-9