Abstract
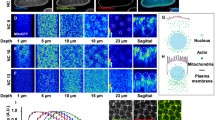
Changes in the distribution of mitochondria in the two-cell mouse embryos preceding the developmental arrest in vitro, caused by a genetically determined “two-cell block in vitro” or genisteine treatment, were examined vitally using the mitochondrial-specific probe rhodamine 123 and conventional fluorescence microscopy. In the former case, serious disturbances in the localization of mitochondria appeared already from the middle of two-cell stage, long before the time corresponding to the 2nd cleavage division. Comparison of the behavior of mitochondria in the embryos successfully developing between the one- and two-cell stages and that in the embryos that ceased to cleave suggests that the developmental arrest was accompanied by aggregation of the mitochondria into clusters. There are many such clusters unlike in the cytoplasm of normally developing embryos. Intracellular localization of clusters observed in the genisteine-treated embryos differed radically from that observed in the embryos blocked in vitro at the two-cell stage.
Similar content being viewed by others
REFERENCES
Abramczuk, J., Solter, D., and Koprowski, H., The Beneficial Effect of EDTA on Development of Mouse One-Cell Embryos in Chemically Defined Medium, Dev. Biol., 1977, vol. 61, pp. 378–383.
Anderson, E., Condon, W., and Sharp, D., A Study of Oogenesis and Early Embryogenesis in the Rabbit, Oryctolagus cuniculus, with Special Reference to the Structural Changes of Mitochondria, J. Morphol., 1971, vol. 130, pp. 67–92.
Barnett, D.K. and Bavister, B.D., What Is the Relationship between the Metabolism of the Preimplantation Embryos and Their Developmental Competence?, Mol. Reprod. Devel., 1996, vol. 43, pp. 105–133.
Barnett, D.K., Kimura, J., and Bavister, B.D., Translocation of Active Mitochondria during Hamster Preimplantation Embryo Development Studied by Confocal Laser Scanning Microscopy, Devel. Dyn., 1996, vol. 205, pp. 64–72.
Batten, B.E., Albertini, D.F., and Ducibella, T., Patterns of Organelle Distribution in Mouse Embryos during Preimplantation Development, Am. J. Anat., 1987, vol. 178, pp. 204–213.
Bavister, B.D. and Squirell, J.M., Mitochondrial Distribution and Function in Oocytes and Early Embryos, Hum. Reprod., 2000, vol. 15, suppl. 2, pp. 189–198.
Biggers, J.D., The Culture of the Mammalian Preimplantation Embryo, Implantation in Mammals, New York: Raven, 1993, pp. 123–135.
Biggers, J.D., Reflections on the Culture of the Preimplantation Embryo, Int. J. Dev. Biol., 1998, vol. 42, pp. 879–884.
Biggers, J.D. and Borland, R.M., Physiological Aspects of the Preimplantation Mammalian Embryo, Annu. Rev. Physiol., 1976, vol. 38, pp. 95–121.
Calarco, P.G. and Brown, E.H., An Ultrastructural and Cytological Study of Preimplantation Development of the Mouse, J. Exp. Zool., 1969, vol. 171, pp. 253–283.
Chatot, C.L., Ziomek, C.A., Bavister, B.D., et al., An Improved Culture Medium Supports Development of Random-Bred 1-Cell Mouse Embryos in vitro, J. Reprod. Fertil., 1989, vol. 86, pp. 679–688.
Choi, Y.H., Zhang, L., Lee, W.H., and Park, K.Y., Genisteine-Induced G2/M Arrest Is Associated with the Inhibition of Cyclin B1 and Induction of p21 in Human Brest Carcinoma Cells, Int. J. Oncol., 1998, vol. 13, pp. 391–396.
Couchman, J.R. and Rees, D.A., Organelle-Cytoskeleton Relationships in Fibroblasts: Mitochondria, Golgi Apparatus, and Endoplasmic Reticulum in the Phases of Movement and Growth, Eur. J. Cell Biol., 1982, vol. 27, pp. 47–54.
David-Ferreira, K.L. and David-Ferreira, J.F., Association between Intermediated-Sized Filaments and Mitochondria in Rat Leudig Cells, Cell Biol. Int. Rep., 1980, vol. 4, pp. 655–662.
Dyban, A.P., Experiments on Mammalian Embryos, Metody biologii razvitiya (Methods of Developmental Biology), Moscow: Nauka, 1974, pp. 217–245.
Gallicano, G.I., Composition, Regulation and Function of the Cytoskeleton in Mammalian Eggs and Embryos, Front. Biosci., 2001, vol. 6, pp. 1089–1108.
Gallicano, G.I., Capco, D.G., and McDaudhey, R.W., Cytoskeletal Sheets Comprised of Intermediate Filaments: Novel Cytoskeletal Elements Characteristic of Mammalian Embryos, J. Cell Biol., 1990, vol. 111, pp. 481–491.
Goddart, M.J. and Pratt, H.P.M., Control of Events during Early Cleavage of the Mouse Embryo: An Analysis of the “2-Cell Block,” J. Embryol. Exp. Morphol., 1983, vol. 73, pp. 111–133.
Hillman, N. and Tasca, R.J., Ultrastructural and Autoradiographic Studies of Mouse Cleavage Stages, Am. J. Anat., 1969, vol. 126, pp. 151–173.
Ho, Y., Wigglesworth, K., Eppig, J.J., and Schults, R.M., Preimplantation Development of Mouse Embryos in KSOM: Augmentation by Amino Acids and Analysis of Gene Expression, Mol. Reprod. Devel., 1995, vol. 41, pp. 232–238.
Kimber, S.J. and Surani, M.A.H., Morphogenetic Analysis of Changing Cell Associations Following Release of 2-Cell and 4-Cell Mouse Embryos from Cleavage Arrest, J. Embryol. Exp. Morphol., 1981, vol. 61, pp. 331–345.
Krisher, R.L. and Bavister, B.D., Correlation of Mitochondrial Organisation with Developmental Competence in Bovine Oocytes Matured in vitro, Biol. Reprod., 1997, vol. 56, suppl. 1, p. 602.
Lawitts, J.A. and Biggers, J.D., Optimization of Mouse Embryo Culture Media Using Simplex Methods, J. Reprod. Fert., 1991, vol. 91, pp. 543–556.
Matsukawa, Y., Marui, N., Sakai, T., et al., Genisteine Arrests Cell Cycle Progression at G2/M, Cancer Res., 1993, vol. 53, pp. 1328–1331.
Matsumoto, H., Shoi, N., Sugavara, M, et al., Microscopic Analysis of Enzyme Activity, Mitochondrial Distribution and Hydrogen Peroxide in Two-Cell Rat Embryos, J. Reprod. Fert., 1998, vol. 113, pp. 231–238.
Motta, P.M., Nottola, S.A., Makabe, S., and Heyn, R., Mitochondrial Morphology in Human Fetal and Adult Gene Cells, Hum. Reprod., 2000, vol. 15, suppl. 2, pp. 129–147.
Muggleton-Harris, A.L. and Brown, J.J., Cytoplasmic Factors Influence Mitochondrial Reorganization and Resumption of Cleavage during Culture of Early Mouse Embryos, Hum. Reprod., 1988, vol. 3, pp. 1020–1028.
Neganova, I.E. and Sekirina, G.G., “Two-Cell Block” and Viability of BALB/c Mouse Embryos after Explantation during the Second Cleavage Cycle, Ontogenez (Moscow), 1996, vol. 27, pp. 371–378.
Neganova, I.E., Sekirina, G.G., and Eichenlaub-Ritter, U., Surface-Expressed E-Cadherin, and Mitochondrial and Microtubule Distribution in Rescue of Mouse Embryos from 2-Cell Block by Aggregation, Mol. Hum. Reprod., 2000, vol. 6, pp. 454–464.
Noto, V., Campo, R., Roziers, P., et al., Mitochondrial Distributions after Fast Embryo Freezing, Hum. Reprod., 1993, vol. 8, pp. 2115–2118.
Pereira, A.J., Dalby, B., Stevart, R.J., et al., Mitochondrial Association of a Plus End-Directed Microtubule Motor Expressed during Mitosis in Drosophila melanogaster, J. Cell Biol., 1997, vol. 136, pp. 1081–1090.
Pratt, H.P.M. and Muggleton-Harris, A.L., Cycling Cytoplasmic Factors That Promote Mitosis in the Cultured 2-Cell Mouse Embryo, Development (Cambridge, UK), 1988, vol. 104, pp. 115–120.
Pratt, H., Experiments with Preimplantation Mouse Embryos, Biologiya razvitiya mlekopitayushchikh. Metody (Biology of Mammalian Development. Methods), Moscow: Mir, 1990, pp. 27–64.
Quinn, P., Barros, C., and Wittingham, D.G., Preservation of Hamster Oocytes to Assay the Fertilizing Capacity of Human Spermatozoa, J. Reprod. Fert., 1982, vol. 66, pp. 161–168.
Robl, J.M., Lohse-Heideman, J.K., and First, N.L., Strain Differences in Early Mouse Embryo Development in vitro: Role of the Nucleus, J. Exp. Zool., 1988, vol. 247, pp. 251–256.
Summerhayes, J., Wong, D., and Chen, L.B., Effect of Microtubules and Intermediate Filaments on Mitochondrial Distribution, J. Cell Sci., 1983, vol. 61, pp. 87–105.
Sun, Q.Y., Wu, G.M., Lai, L., et al., Translocation of Active Mitochondria during Pig Oocyte Maturation, Fertilisation, and Early Embryo Development in vitro, Reproduction, 2001, vol. 122, pp. 155–163.
Sutovsky, P., Navara, C.S., and Shatten, G., Fate of the Sperm Mitochondria and the Incorporation, Conversion and Disassembly of the Sperm Tail Structures during Bovine Fertilisation, Biol. Reprod., 1996, vol. 55, no. 6, pp. 11195–11205.
Suzuki, S., Komatsu, S., Kitai, H., et al., Analysis of Cytoplasmic Factors in Development Cleavage of Mouse Embryo, Cell Differ., 1988, vol. 24, pp. 133–138.
Tokura, T., Noda, Y., Goto, Y., and Mori, T., Sequential Observation of Mitochondrial Distribution in Mouse Oocytes and Embryos, J. Assist. Reprod. Genet., 1993, vol. 10, pp. 417–426.
Van Blerkom, J., Runner, H., and Meredith, N., Mitochondrial Reorganization during Resumption of Arrested Meiosis in the Mouse Oocyte, Am. J. Anat., 1984, vol. 171, pp. 335–355.
Van Blerkom, J., Davis, P., and Alexander, S., Differential Mitochondrial Distribution in Human Pronuclear Embryos Leads to Disproportionate Inheritance between Blastomeres: Relationship to Microtubular Organisation, ATP Content, and Competence, Hum. Reprod., 2000, vol. 15, no. 12, pp. 2621–2633.
Whitten, W.K. and Biggers, J.D., Complete Development in vitro of the Preimplantation Stages of the Mouse in a Simple Chemically Defined Medium, J. Reprod. Fert., 1968, vol. 17, pp. 399–401.
Author information
Authors and Affiliations
Additional information
Translated from Ontogenez, Vol. 36, No. 1, 2005, pp. 51–60.
Original Russian Text Copyright © 2005 by Bogolyubova.
Rights and permissions
About this article
Cite this article
Bogolyubova, N.A. Changes in the distribution of mitochondria in mouse embryos blocked at the two-cell stage. Russ J Dev Biol 36, 43–50 (2005). https://doi.org/10.1007/s11174-005-0007-9
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/s11174-005-0007-9