Abstract
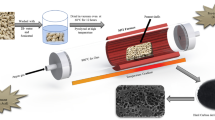
The microporous carbon adsorbent with the specific micropore volume W0 = 0.80 cm3 g−1, micropore width X0 = 1.32 nm, and specific surface area SBET = 2115 m2 g−1 was synthesized by the thermochemical activation method from the carbonizate of nutshell Macadamia in the presence of orthophosphoric acid at 1173 K. At 303 K and 20 MPa, the adsorption capacity of the adsorbent to methane reaches ∼22 wt.%. The volume density of adsorbed methane on the compacted carbon adsorbent with the binder at a pressure of 10 MPa was found to be ~200 nm3 m−3.*
Similar content being viewed by others
Change history
31 January 2024
An Erratum to this paper has been published: https://doi.org/10.1007/s11172-023-4117-2
References
Handbook of Air Pollution Technology, Eds S. Calvert, H. M. Englund, Wiley-Interscience, New York, 1984, 1066 pp.; DOI: https://doi.org/10.1201/M9286.
G. K. Lavrenchenko, Tekhnicheskie Gazy [Technical Gases], 2006, No. 5, 2 (in Russian).
A. A. Fomkin, A. Yu. Tsivadze, A. V. Shkolin, V. M. Mukhin, V. I. Dudarev, Protection of Metals and Physical Chemistry of Surfaces, 2014, 50, 689–693; DOI: https://doi.org/10.7868/S0044185614060035.
E. M. Strizhenov, A. A. Fomkin, A. A. Zherdev, A. A. Pribylov, Fizikokhimiya poverkhnosti i zashchita materialov [Physicochemistry of Surface and Protection of Materials], 2012, 48, 521 (in Russian).
I. E. Men’shchikov, A. A. Fomkin, A. V. Shkolin, V. Yu. Yakovlev, E. V. Khozina, Russ. Chem. Bull., 2018, 67, 1814–1822; DOI: https://doi.org/10.1007/s11172-018-2294-1.
M. M. Dubinin, Progress Surface Membrane Sci., 1975, 9, 1–70.
S. J. Gregg, K. S. W. Sing, Adsorption, Surface Area and Porosity, Academic Press Publ., London–New York, 1967, 371 pp.; DOI: https://doi.org/10.1002/bbpc.19820861019.
Eksperimental’nye metody v adsorbtsii i khromatografi i [Experimental Methods in Adsorption and Chromatography], Eds A. V. Kiselev and V. P. Dreving, Izd. Mos. Gos. Univ., Moscow, 1973 (in Russian).
K. M. Anuchin, A. A. Fomkin, A. P. Korotych, A. M. Tolmachev, Fizikokhimiya poverkhnosti i zashchita materialov [Physicochemistry of Surface and Protection of Materials], 2014, 50, 156–160; DOI: https://doi.org/10.7868/S004418561402003X (in Russian).
V. V. Sychev, A. A. Vasserman, V. A. Zagoruchenko, A. D. Kozlov, G. A. Spiridonov, V. A. Tsymarnyi, Termodinamicheskie svoistva metana [Thermodynamic Properties of Methane], Izd. Standartov, Moscow, 1979 (in Russian).
A. A. Pribylov, S. M. Kalashnikov, V. V. Serpinskii, Bull. Acad. Sci. USSR, Div. Chem. Sci., 1990, 39, 1105; DOI: https://doi.org/10.1007/BF00962364.
V. A. Bakaev, Bull. Acad. Sci. USSR, Div. Chem. Sci., 1971, 20, 2516; DOI: https://doi.org/10.1007/BF00853614.
A. A. Fomkin, Adsorption, 2005, 11, 425; DOI: https://doi.org/10.1007/s10450-005-5636-x.
A. V. Shkolin, A. A. Fomkin, Kolloid. Zh. [ColloidalJ.], 2009, 71, 116 (in Russian).
A. V. Shkolin, A. A. Fomkin, Russ. Chem. Bull., 2009, 58, 717–721; DOI: https://doi.org/10.1007/s11172-009-0083-6.
A. I. Vlasov, V. A. Bakaev, M. M. Dubinin, V. V. Serpinskii, Dokl. Akad. Nauk SSSR [Reports of USSR Acad. Sci.], 1981, 260, 904–906 (in Russian).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The authors declare no competing interests.
Additional information
This work was carried out within the framework of the state assignment of the Ministry of Science and Higher Education of the Russian Federation (theme No. 122011300053-8) with financial support of the Institute of Environmental Technology of the Vietnam Academy of Science and Technology (Grant No. CSCL.01/22-23) in accord with the plan of the Scientific Council on Physical Chemistry of the Russian Academy of Sciences (theme No. 23-03-460-01).
No human or animal subjects were used in this research.
The amount of methane (m3) in 1 m3 of the storage system at a normal temperature of 293 K and a pressure of 101 kPa is indicated.
Published in Russian in Izvestiya Akademii Nauk. Seriya Khimicheskaya, Vol. 72, No. 9, pp. 2025–2030, September, 2023.
Rights and permissions
About this article
Cite this article
Tuyen, N.D., Pribylov, A.A., Fomkin, A.A. et al. Synthesis of active carbon from nutshell Macadamia and its adsorption affinity toward methane. Russ Chem Bull 72, 2025–2030 (2023). https://doi.org/10.1007/s11172-023-3995-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11172-023-3995-7