Abstract
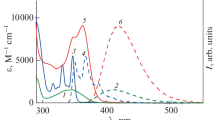
A method for the synthesis of symmetric biphotochromic dyads from 8-hydroxyquin-aldine, arylaldehydes, and 1,10-dibromodecane was developed. The dyads comprise two identical arylvinyl-8-oxyquinoline fragments (Ar = Ph, anthracen-9-yl, pyren-1-yl) covalently linked by a decamethylene chain. The structure of the dyads was studied in terms of the density functional theory using the M06-2X hybrid functional. Calculations predict the existence of dyad conformers where n-stacking interaction promotes the approach of terminal photochromes to distances sufficient for the [2 + 2]-photocycloaddition reaction to occur. The luminescent properties of the dyads were studied. The dyads with phenyl and pyrenyl groups were found to be relatively good luminophores with the fluorescence quantum yields φfl ⩾ 0.15 (cf. a low φfl value of 0.022 for the dyad bearing the anthryl group).
Similar content being viewed by others
References
V. Z. Shirinian, D. V. Lonshakov, A. G. Lvov, M. M. Krayushkin, Russ. Chem. Rev., 2013, 82, 511; DOI: https://doi.org/10.1070/RC2013v082n06ABEH004339.
E. V. Nosova, S. Achelle, G. N. Lipunova, V. N. Charushin, O. N. Chupakhin, Russ. Chem. Rev., 2019, 88, 1128; DOI: https://doi.org/10.1070/RCR4887.
C. Yun, J. You, J. Kim, J. Huh, E. Kim, J. Photochem. Photobiol. C: Photochem. Rev., 2009, 10, 111; DOI: https://doi.org/10.1016/j.jphotochemrev.2009.05.002.
J. Cusido, E. Deniz, F. M. Raymo, Eur. J. Org. Chem., 2009, 2031; DOI: https://doi.org/10.1002/ejoc.200801244.
T. Fukaminato, S. Ishida, R. Métivier, NPG Asia Materials, 2018, 10, 859; DOI: https://doi.org/10.1038/s41427-018-0075-9.
A. F. Khasanov, D. S. Kopchuk, I. L. Nikonov, O. S. Taniya, I. S. Kovalev, G. V. Zyryanov, V. L. Rusinov, O. N. Chupakhin, Russ. Chem. Bull., 2021, 70, 999; DOI: https://doi.org/10.1007/s11172-021-3179-2.
L. S. Hung, C. H. Chen, Mater. Sci. Eng., 2002, R39, 143; DOI: https://doi.org/10.1016/S0927-796X(02)00093-1.
C. H. Chen, J. Shi, Coord. Chem. Rev., 1998, 171, 161; DOI: https://doi.org/10.1016/S0010-8545(98)90027-3.
K. Ch. Song, J. S. Kim, S. M. Park, K.-Ch. Chung, S. Ahn, S.-K. Chang, Org. Lett., 2006, 8, 3413; DOI: https://doi.org/10.1021/ol060788b.
Y.-W. Shi, M.-M. Shi, J.-C. Huang, H.-Z. Chen, M. Wang, X.-D. Liu, Y.-G. Ma, H. Xu, B. Yang, Chem. Commun., 2006, 1941; DOI: https://doi.org/10.1039/B516757D.
J. Liang, Q. Wei, Ch. Qiao, Zh. Xia, G. Ye, S. Chen, Chin. J. Chem., 2012, 30, 715; DOI: https://doi.org/10.1002/cjoc.201280017.
S. Sehlangia, M. Devi, N. Nayak, N. Garg, A. Dhir, C. P. Pradeep, ChemistrySelect, 2020, 5, 5429; DOI: https://doi.org/10.1002/slct.202000674.
M. Hadavand, M. R. Jafari, F. Pakpour, D. Ghanbari, J. Nanopart. Res., 2021, 23, 61; DOI: https://doi.org/10.1007/s11051-021-05174-9.
W.-G. Jia, X.-T. Zhi, X.-D. Li, J.-P. Zhou, R. Zhong, H. Yu, R. Lee, Inorg. Chem., 2021, 60, 4313; DOI: https://doi.org/10.1021/acs.inorgchem.1c0005.
O. V. Serdyuk, I. V. Evseenko, G. A. Dushenko, Yu. V. Revinskii, I. E. Mihailov, Russ. J. Org. Chem., 2012, 48, 78; DOI: https://doi.org/10.1134/S1070428012010113.
O. V. Chashchikhin, M. F. Budyka, T. N. Gavrishova, P. A. Nikulin, Chem. Phys. Lett., 2018, 696, 135; DOI: https://doi.org/10.1016/j.cplett.2018.02.055.
Yu. P. Kovtun, Ya. A. Prostota, A. I. Tolmachev, Zh. nauch. prikl. fotografi i [J. Sci. Appl. Photography Cinematography], 2000, 45, 51 (in Russian).
M. F. Budyka, N. I. Potashova, T. N. Gavrishova, V. M. Li, Nanotechnol. Russ., 2012, 7, 280; DOI: https://doi.org/10.1134/S1995078012030032.
M. F. Budyka, Russ. Chem. Rev., 2017, 86, 181; DOI: 10.1070/RCR4657.
M. F. Budyka, N. I. Potashova, T. N. Gavrishova, V. M. Li, High Energy Chem., 2008, 42, 446; DOI: https://doi.org/10.1134/S0018143908060052.
M. F. Budyka, N. I. Potashova, T. N. Gavrishova, V. M. Li, High Energy Chem., 2014, 48, 185; DOI: https://doi.org/10.1134/S0018143914030047.
M. F. Budyka, V. M. Li, Photochem. Photobiol. Sci., 2018, 17, 213; DOI: https://doi.org/10.1039/C7PP00359E.
M. F. Budyka, V. M. Li, Russ. Chem. Bull., 2021, 70, 1665; DOI: https://doi.org/10.1007/s11172-021-3268-2.
M. F. Budyka, N. I. Potashova, T. N. Gavrishova, V. M. Lee, High Energy Chem., 2012, 46, 309; DOI: https://doi.org/10.1134/S0018143912040054.
M. F. Budyka, T. N. Gavrishova, N. I. Potashova, A. V. Chernyak, Mendeleev Commun., 2015, 25, 106; DOI: https://doi.org/10.1016/j.mencom.2015.03.008.
E. N. Ushakov, A. I. Vedernikov, N. A. Lobova, S. N. Dmitrieva, L. G. Kuz’mina, A. A. Moiseeva, J. A. K. Howard, M. V. Alfimov, S. P. Gromov, J. Phys. Chem. A, 2015, 119, 13025; DOI: https://doi.org/10.1021/acs.jpca.5b10758.
E. N. Ushakov, S. P. Gromov, Russ. Chem. Rev., 2015, 84, 787; DOI: https://doi.org/10.1070/RCR4514.
X. Ling, H. Zeng, Youji Huaxue, 2009, 29, 742.
Xi. Ouyang, H. Zeng, W. Ji, J. Phys. Chem. B, 2009, 113, 14565; DOI: https://doi.org/10.1021/jp905390q.
M. Normand-Bayle, C. Bénard, F. Zouhiri, J.-F. Mouscadet, H. Leh, C.-M. Thomas, G. Mbemba, D. Desmaële, J. d’Angelo, Bioorg. Med. Chem. Lett., 2005, 15, 4019; DOI: https://doi.org/10.1016/j.bmcl.2005.06.036.
Ya. Huo, Z. Li, Ju. Li, T. Kong, H. Liang, G. Guo, Ch. Pan, S. Wang, Pat. CN 106496117, Chem. Abstrs, 2017, 166, 362598.
Ji. Xiong, Z. Li, Ji. Tan, Sh. Ji, Ji. Sun, Xi. Li, Ya. Huo, Analyst, 2018, 143, 4870; DOI: https://doi.org/10.1039/C8AN00940F.
Zh. Wang, Ya. Wang, Bo Wang, W. Li, L. Huang, Xi. Li, J. Med. Chem., 2015, 58, 8616; DOI: https://doi.org/10.1021/jm401047q.
F.-S. Chang, W. Chen, Ch. Wang, Ch.-Ch. Tzeng, Y.-L. Chen, Bioorg. Med. Chem., 2010, 18, 124; DOI: https://doi.org/10.1016/j.bmc.2009.11.012.
M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery, Jr., J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, O. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski, D. J. Fox, Gaussian 09, Revision B.01, Gaussian, Inc., Wallingford CT, 2010.
U. Mazzucato, F. Momicchioli, Chem. Rev., 1991, 91, 1679; DOI: https://doi.org/10.1021/cr00008a002.
M. F. Budyka, I. V. Oshkin, Int. J. Quantum Chem., 2011, 111, 3673; DOI: https://doi.org/10.1002/qua.22797.
M. F. Budyka, T. N. Gavrishova, V. M. Li, S. A. Dozmorov, High Energy Chem., 2019, 53, 5; DOI: https://doi.org/10.1134/S0018143919010028.
J. Catalan, Chem. Phys. Lett., 2006, 421, 134; DOI: https://doi.org/10.1016/j.cplett.2006.01.077.
J. W. Chung, Y. You, H. S. Huh, B.-K. An, S.-J. Yoon, S. H. Kim, S. W. Lee, S. Y. Park, J. Am. Chem. Soc., 2009, 131, 8163; DOI: https://doi.org/10.1021/ja900803d.
M. F. Budyka, Russ. Chem. Rev., 2012, 81, 477; DOI: https://doi.org/10.1070/RC2012v081n06ABEH004274.
G. Galiazzo, P. Bortolus, G. Gennari, Gazz. Chim. Ital., 1990, 120, 581.
M. F. Budyka, N. I. Potashova, T. N. Gavrishova, V. M. Li, V. Yu. Gak, I. A. Grineva, High Energy Chem., 2018, 52, 222; DOI: https://doi.org/10.1134/S0018143918030062.
V. Kozlovski, V. Brusov, I. Sulimenkov, A. Pikhtelev, A. Dodonov, Rapid Commun. Mass Spectrom., 2004, 18, 780; DOI: https://doi.org/10.1002/rcm.1405.
H.-D. Becker, Chem. Rev., 1993, 93, 145; DOI: https://doi.org/10.1021/cr00017a008.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare no competing interests.
Additional information
This work was performed using facilities at the Multiple-User Analytical Center, Federal Research Center of Problems of Chemical Physics and Medicinal Chemistry, Russian Academy of Sciences.
This work was carried out within the framework of the State Assignment (State Reg. No. AAAA-A19-119070790003-7).
No human or animal subjects were used in this research.
Published in Russian in Izvestiya AkademiiNauk. Seriya Khimicheskaya, Vol. 72, No. 9, pp. 2013–2024, September, 2023.
Rights and permissions
About this article
Cite this article
Budyka, M.F., Gavrishova, T.N., Li, V.M. et al. Biphotochromic dyads based on aryl-8-oxyquinolylethylenes with a decamethylene bridge: the synthesis, structure, spectral, and luminescent properties. Russ Chem Bull 72, 2013–2024 (2023). https://doi.org/10.1007/s11172-023-3994-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11172-023-3994-8