Abstract
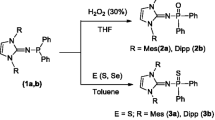
The synthesis of a series of new organophosphorane bis-ylide ligands is described. Upon their deprotonation, the stabilization of the ylide α-carbanion is achieved through the negative charge delocalization either over the π-system of the α-aryl groups bonded to carbanionic centers or over the {Cp}-type aromatic system in the case when the α-carbanion is involved in this system. The dibenzyl ylides [Ph2P(=CHAr)(CH2Ar)] were synthesized in situ starting from Ph2PLi and 2 equiv. of benzyl halides that significantly increased the yields of the target compounds. Ligands of the fluorenylidene, indenylidene, and cyclopentadienylidene types were synthesized in high yields. π-Stabilized organo-phosphorane ligands are readily metalated with potassium hydride and sodium amide to give the corresponding salts as solvates with THF. The reaction of 9-(diphenylphosphino)-fluorenide, Li[Ph2PC13H8], with Ph2PCl afforded the gem-bis(phosphine) ligand [9,9-(Ph2P)2C13H8] that readily gave the corresponding palladium complex [{κ-P, P-(Ph2P)2C13H8}PdCl2] in high yield.
Similar content being viewed by others
References
A. William Jonson, Ylides and imines of phosphorus, John Willey & Sons, Inc., Canada, 1993, 587.
H. Staudinger, J. Meyer, Helv. Chim. Acta, 1919, 2, 636; DOI: https://doi.org/10.1002/hlca.19190020164.
G. Wittig, G. Geissler, Ann. Chem., 1953, 580, 44; DOI: https://doi.org/10.1002/jlac.19535800107.
G. Wittig, L. Schollkopf, Chem. Ber., 1954, 87, 1318; DOI: https://doi.org/10.1002/cber.19540870919.
H. Schmidbaur, Acc. Chem. Res., 1975, 8, 62; DOI: https://doi.org/10.1021/ar50086a003.
H. Schmidbaur, W. Scharf, H.-J. Füller, Z. Naturforsch. Teil B, 1977, 32, 858; DOI: https://doi.org/10.1515/znb-1977-0805.
W. C. Kaska, Coord. Chem. Rev., 1983, 48, l; DOI: https://doi.org/10.1016/0010-8545(83)85001-2.
E. P. Urriolabeittia, Top. Organomet. Chem., 2010, 30, 15; DOI: https://doi.org/10.1007/978-3-642-04722-0_2.
E. G. McKenna, B. J. Walker, Tetrahedron Lett., 1988, 29, 485; DOI: https://doi.org/10.1016/s0040-4039(00)80128-8.
H-J. Cristau, Y. Ribeill, F. Plenat, L. Chiche, Phosphorus and Sulfur, 1987, 30, 135; DOI: https://doi.org/10.1080/03086648708080540.
K. A. Rufanov, B. Ziemer, M. Hummert, S. Schutte, Eur. J. Inorg. Chem., 2004, 24, 4759; DOI: https://doi.org/10.1002/ejic.200400664.
K. A. Rufanov, A. Spannenberg, Mendeleev Commun., 2008, 18, 32; DOI: https://doi.org/10.1016/j.mencom.2008.01.013.
S. Harder, M. Lutz, Organometallics, 1997, 16, 225; DOI: https://doi.org/10.1021/om9605443.
P. J. Bailey, T. Barrett, S. Parsons, J. Organomet. Chem., 2001, 625, 236; DOI: 0.1016/s0022-328x(01)00674-x.
R. Geitner, I. Kosygin, H. Görls, J. Pahl, S. Harder, M. Westerhausen, J. Langer, J. Coord. Chem., 2015, 68, 3302; DOI: https://doi.org/10.1080/00958972.2015.1072625.
Y. Matsusaka, S. Shitaya, K. Nomura, A. Inagaki, Inorg. Chem., 2017, 56, 1027; DOI: https://doi.org/10.1021/acs.inorgchem.6b02423.
L. Baiget, M. Bouslikhane, J. Escudie, G. C. Nemes, Phosphorus, Sulfur, Silicon Relat. Elem., 2003, 178, 1949; DOI: https://doi.org/10.1080/10426500390228639.
F. Schroeder, J. Sundermeyer, Organometallics, 2015, 34, 1017; DOI: https://doi.org/10.1021/om501325h.
M. Shahid, I.-ud-Din, M. Mazhar, M. Zeller, A. D. Hunter, Acta Cryst., 2009, E65, m158; DOI: https://doi.org/10.1107/S1600536808042980.
W. L. Steffen, G. J. Palenik, Inorg. Chem., 1976, 15, 2432; DOI: https://doi.org/10.1021/ic50164a025.
J. V. Barkley, J. C. Grimshaw, S. J. Higgins, P. B. Hoare, M. K. McCart, A. K. Smith, J. Chem. Soc. Dalton Trans., 1995, 2901; DOI: https://doi.org/10.1039/DT9950002901.
F. Mathey, J. P. Lampin, Tetrahedron, 1975, 31, 2685; DOI
Stoe & Cie GmbH, IPDS Software, 1996X-AREA and X-RED32, Darmstadt, Germany, 2006.
Bruker AXS GmbH, APEX 2, Karlsruhe, Germany, 2012.
G. M. Sheldrick, Acta. Crystallogr. Sect C., 2015, 71, 3; DOI: https://doi.org/10.1107/S2053229614024218.
G. Sheldrick, Acta Cryst. A, 2008, 64, 112; DOI: https://doi.org/10.1107/S0108767307043930.
Author information
Authors and Affiliations
Corresponding author
Additional information
The study was financially supported by the Immanuel Kant Baltic Federal University within the framework of the research project No. 877. We are grateful to Dr. A. Spannenberg (Leibniz Institute for Catalysis (LIKAT Rostock)) for performing X-ray diffraction experiments and the structure solution of 2c, Dr. P. Neumueller (Department of Chemistry, Humboldt University of Berlin) for performing X-ray diffraction experiments and the structure solution of 12, and the stuff of the X-ray Service Department of the Department of Chemistry of the Philipps University of Marburg for performing X-ray diffraction experiments and the structure solution of 18, 23, and 25.
Published in Russian in Izvestiya Akademii Nauk. Seriya Khimicheskaya, Vol. 72, No. 6, pp. 1438–1453, June, 2023.
Rights and permissions
About this article
Cite this article
Rufanov, K.A., Shevelyukhina, A.V. Synthesis of new π-stabilized organophosphorane bis-ylide ligands. Russ Chem Bull 72, 1438–1453 (2023). https://doi.org/10.1007/s11172-023-3919-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11172-023-3919-6