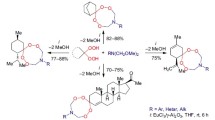
Abstract
Cyclocondensation reaction of linear α,ω-diols (C3, C5, C6, and C8) with 1,1-dihydroperoxycycloalkanes and formaldehyde with the participation of lanthanide catalysts affords spiromacrocyclic diperoxides.
Similar content being viewed by others
References
D. A. Casteel, Nat. Prod. Rep., 1992, 9, 289; DOI: https://doi.org/10.1039/NP9920900289.
K. J. McCullough, M. Nojima, Curr. Org. Chem., 2001, 5, 601; DOI: https://doi.org/10.2174/1385272013375346.
D. A. Casteel, Nat. Prod. Rep., 1999, 16, 55; DOI: https://doi.org/10.1039/A705725C.
M. Bu, B. B. Yang, L. Hu, Curr. Med. Chem., 2016, 23, 383; DOI: https://doi.org/10.2174/0929867323666151127200949.
A. Robert, R. A. Meunier, Chem. Soc. Rev., 1998, 27, 273; DOI: https://doi.org/10.1039/A827273Z.
J. L. Vennerstrom, H.-N. Fu, W. Y. Ellis, Jr. A. L. Ager, J. K. Wood, S. L. Andersen, L. Gerena, W. K. Milhous, J. Med. Chem., 1992, 35, 3023; DOI: https://doi.org/10.1039/A900826H.
G. H. Posner, H. O’Dowd, T. Caferro, J. N. Cumming, P. Ploypradith, S. Xie, T. A. Shapiro, Tetrahedron Lett., 1998, 39, 2273–2276; DOI: https://doi.org/10.1016/S0040-4039(98)00290-1.
P. H. Dussault, K. Woller, J. Am. Chem. Soc., 1997, 119, 3824–3825; DOI: https://doi.org/10.1021/ja970174p.
P. M. O’Neill, N. L. Searle, K. J. Raynes, J. L. Maggs, S. A. Ward, R. C. Storr, B. K. Park, G. H. Posner, Tetrahedron Lett., 1998, 39, 6065–6068; DOI: https://doi.org/10.1016/S0040-4039(98)01248-9.
S. Fielder, D. D. Rowan, M. S. Sherburn, Tetrahedron., 1998, 54, 12907–12922; DOI: https://doi.org/10.1016/S0040-4020(98)00782-0.
H.-S. Kim, Y. Nagai, K. Ono, K. Begum, Y. Wataya, Y. Hamada, K. Tsuchiya, A. Masuyama, M. Nojima, K. McCullough, J. Med. Chem., 2001, 44, 2357–2361; DOI: https://doi.org/10.1021/jm010026g.
K. J. McCullough, T. Ito, T. Tokuyasu, A. Masuyama, M. Nojima, Tetrahedron Lett., 2001, 42, 5529–5532; DOI: https://doi.org/10.1016/S0040-4039(01)01015-2.
T. Ito, T. Tokuyasu, A. Masuyama, M. Nojimaa, K. J. McCullough, Tetrahedron, 2003, 59, 525–536; DOI: https://doi.org/10.1016/S0040-4020(02)01556-9.
K. J. McCullough, H. Tokuhara, A. Masuyama, M. Nojima, Org. Biomol. Chem., 2003, 1, 1522–1527; DOI: https://doi.org/10.1039/B300342F.
Y. Nonami, Y. Ushigoe, A. Masuyama, M. Nojima, K. J. McCullough, Tetrahedron Lett., 1998, 39, 6597–6600; DOI: https://doi.org/10.1016/S0040-4039(98)01375-6.
K. J. McCullough, Y. Nonami, A. Masuyama, M. Nojima, H.-S. Kim, Y. Wataya, Tetrahedron Lett., 1999, 40, 9151–9155; DOI: https://doi.org/10.1016/S0040-4039(99)01944-9.
A. V. Arzumanyan, R. A. Novikov, A. O. Terent’ev, M. M. Platonov, V. G. Lakhtin, D. E. Arkhipov, A. A. Korlyukov, V. V. Chernyshev, A. N. Fitch, A. T. Zdvizhkov, I. B. Krylov, Y. V. Tomilov, G. I. Nikishin, Organometallics, 2014, 33, 2230–2246; DOI: https://doi.org/10.1021/om500095x.
A. V. Arzumanyan, A. O. Terent’ev, R. A. Novikov, V. G. Lakhtin, V. V. Chernyshev, A. N. Fitch, G. I. Nikishin, Eur. J. Org. Chem., 2014, 31, 6877–6883; DOI: https://doi.org/10.1002/ejoc.201402895.
N. N. Makhmudiyarova, I. R. Ishmukhametova, T. V. Tyumkina, A. G. Ibragimov, U. M. Dzhemilev, Tetrahedron Lett., 2018, 59, 3161–3164; DOI: https://doi.org/10.1016/j.tetlet.2018.07.010.
N. N. Makhmudiyarova, G. M. Khatmullina, R. Sh. Rakhimov, E. S. Meshcheryakova, A. G. Ibragimov, U. M. Dzhemilev, Tetrahedron, 2016, 72, 3277–3281; DOI: https://doi.org/10.1016/j.tet.2016.04.055.
N. N. Makhmudiyarova, I. R. Ishmukhametova, L. U. Dzhemileva, T. V. Tyumkina, V. A. D’yakonov, A. G. Ibragimov, U. M. Dzhemilev, RSC Adv., 2019, 9, 18923–18929; DOI: https://doi.org/10.1039/C9RA02950H.
N. N. Makhmudiyarova, I. R. Ishmukhametova, L. U. Dzhemileva, V. A. D’yakonov, A. G. Ibragimov, U. M. Dzhemilev, Molecules, 2020, 25, 1874; DOI: https://doi.org/10.3390/molecules25081874.
N. N. Makhmudiyarova, I. R. Ishmukhametova, A. G. Ibragimov, U. M. Dhzemilev, Dokl. Chem., 2020, 492, 93–100; DOI: https://doi.org/10.31857/S2686953520040044.
I. Ugi, S. Heck, Comb. Chem. High Throughput Screen., 2001, 4, 1–34; DOI: https://doi.org/10.2174/1386207013331291.
P. A. Grieco, A. Bahsas, Tetrahedron Lett., 1988, 29, 5855–5858; DOI: https://doi.org/10.1016/S0040-4039(00)82208-X.
B. M. Trost, Science, 1991, 254, 1471–1477; DOI: https://doi.org/10.1126/science.1962206.
N. N. Makhmudiyarova, I. R. Ishmukhametova, K. R. Shangaraev, L. U. Dzhemileva, V. A. D’yakonov, A. G. Ibragimov, U. M. Dzhemilev, N. J. Chem., 2021, 45, 2069–2077; DOI: https://doi.org/10.1039/D0NJ05511E.
S. Oda, J. Franke, M. Krishce, J. Chem. Sci., 2016, 7, 136–141; DOI: https://doi.org/10.1039/C5SC03854E.
U. Wellmar, J. Heterocycl. Chem., 1998, 35, 1531–1532; DOI: https://doi.org/10.1002/jhet.5570350653.
K. Krohn, S. Cludius-Brandt, Synthesis, 2010, 8, 1344–1348; DOI: https://doi.org/10.1055/s-0029-1218658.
S. Vojacek, K. Beese, Z. Alhalabi, S. Swyter, A. Bodtke, C. Carola Schulzke, M. Jung, W. Sippl, A. Link, Arch. Pharm., 2017, 350, e1700097; DOI: https://doi.org/10.1002/ardp.201700097.
H. Liangjie, W. Dan, X. Weilan, C. Jing, Z. Zuoxiang, China Pat. CN102086203 A; 2011.
A. O. Terent’ev, M. M. Platonov, Y. N. Ogibin, G. I. Nikishin, Synthetic Commun., 2007, 37, 1281–1287; DOI: https://doi.org/10.1080/00397910701226384.
Author information
Authors and Affiliations
Corresponding author
Additional information
Dedicated to Academician of the Russian Academy of Sciences I. P. Beletskaya on the occasion of her anniversary.
The study was financially supported by the Russian Foundation for Basic Research (Project No. 22-13-00202). Synthesis of 12-membered macrocyclic diperoxides was carried out as a part of the state task of the Ministry of Science and Higher Education of the Russian Federation (No. FMRS-2022-0079).
No human or animal subjects were used in this research.
The authors declare no competing interests.
Published in Russian in Izvestiya Akademii Nauk. Seriya Khimicheskaya, Vol. 72, No. 5, pp. 1161–1165, May, 2023.
Rights and permissions
About this article
Cite this article
Mahmudiyarova, N.N., Ishmukhametova, I.R. & Dzhemilev, U.M. Catalytic synthesis of spiromacrocyclic diperoxides based on α,ω-diols. Russ Chem Bull 72, 1161–1165 (2023). https://doi.org/10.1007/s11172-023-3884-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11172-023-3884-0