Abstract
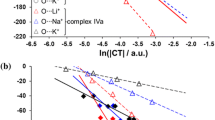
The temperature and concentration dependences of the chemical shift of the phenolic hydroxyl proton in 2,6-diisobornyl-4-methylphenol (1) and 2,6-di-tert-butyl-4-methyl-phenol (ionol, 2) were measured by 1H NMR spectroscopy in the hydrogen bond acceptor (HBA) solvents (diethyl ether, acetone, ethyl acetate). The equilibrium constants, K, of the formation of hydrogen-bonded phenol—solvent complexes were determined and the thermodynamic parameters ΔH° and ΔS° of the complexes were calculated. It was found that the K values of 1 are higher than those of 2 by a factor of about 1.3 in diethyl ether and by a factor of 1.8 in other solvents. The enthalpies of complexation of all systems studied lie in a narrow range of −(12.9−15.5) kJ mol−1. The results of density functional theory calculations of the complex structures suggest that low K values are due to effective shielding of the OH group in 1 by the isobornyl groups. Steric hindrances force the H atom to deviate from the aromatic ring plane to form a hydrogen bond with the O atom of the HBA solvent. The torsion angle α between the C-O-H plane and the ring plane is in the range of 57–70°. Steric hindrances created by the ortho-substituents in ionol are even more pronounced (α ≈ 90°).
Similar content being viewed by others
References
I. Yu. Chukicheva, A. V. Kutchin, Ros. khim. zhurn. [Russ. Chem. J.], 2004, 48, No. 3, 21 (in Russian).
A. F. Gogotov, I. Yu. Chukicheva, A. A. Levchuk, E. V. Buravlev, Do T’em Taj, I. I. Batura, A. V. Kutchin, Khim. rastitel’nogo syr’ya [Chem. Plant Raw Materials], 2011, No. 4, 287 (in Russian).
A. V. Kutchin, A. A. Koroleva, I. V. Fedorova, O. A. Shumova, I. Yu. Chukicheva, Izv. Ufimskogo nauchnogo tsentra RAN[Proc. Ufa Scientific Centre of the RAS], 2012, No. 4, 80 (in Russian).
M. B. Plotnikov, G. A. Chernysheva, V. I. Smolyakova, I. S. Ivanov, A. V. Kutchin, I. Yu. Chukicheva, E. A. Krasnov, Vestn. RAMN [Annals of the Russian Academy of Medical Sciences], 2009, No. 11, 12 (in Russian)
G. A. Chernysheva, V. I. Smolyakova, M. B. Plotnikov, E. A. Yanovskaya, R. V. Gurto, V. V. Udut, I. Yu. Chukicheva, A. V. Kutchin, Eksperim. i klin. farmakologiya [Exp. Clin. Pharmacology], 2011, 74, No. 9, 20 (in Russian).
M. B. Plotnikov, V. I. Smolyakova, I. S. Ivanov, A. V. Kutchin, I. Yu. Chukicheva, E. V. Buravlev, E. A. Krasnov, Pharm. Chem. J., 2010, 44, 530; DOI: https://doi.org/10.1007/s11094-011-0511-4.
L. I. Mazaletskaya, N. I. Sheludchenko, L. N. Shishkina, A. V. Kuchin, I. V. Fedorova, I. Yu. Chukicheva, Petroleum Chemistry (Engl. Transl.), 2011, 51, 348; DOI: https://doi.org/10.1134/S0965544111050100.
L. I. Mazaletskaya, N. I. Sheludchenko, L. N. Shishkina, A. V. Kutchin, I. V. Fedorova, I. Yu. Chukicheva, Russ. J. Phys. Chem. A, 2012, 86, 929; DOI: https://doi.org/10.1134/S0036024412050238.
L. I. Mazaletskaya, N. I. Sheludchenko, L. N. Shishkina, E. V. Buravlev, I. Yu. Chukicheva, A. V. Kutchin, Russ. J. Phys. Chem. A, 2013, 87, 565; DOI: https://doi.org/10.1134/S0036024413040171.
A. Y. Wageeh, A. R. Noorsaadah, A. Ariffin, Bee Abd H. Sharifah, A. A. Abeer, A. K. Farkaad, M. Yaeghoobi, Eur. J. Med. Chem., 2015, 101, 295; DOI: https://doi.org/10.1016/j.ejmech.2015.06.026.
M. A. Polovinkina, M. N. Kolyada, V. P. Osipova, N. T. Berberova, I. Yu. Chukicheva, O. A. Shumova, A. V. Kutchin, Dokl. Chem. (Engl. Transl.), 2019, 484, 48; DOI: https://doi.org/10.1134/S001250081902006X.
E. V. Buravlev, I. V. Fedorova, O. G. Shevchenko, A. V. Kutchin, Russ. Chem. Bull., 2020, 69, 1573; DOI: https://doi.org/10.1007/s11172-020-2937-x.
E. V. Buravlev, O. G. Shevchenko, A. V. Kutchin, Russ. Chem. Bull., 2021, 70, 183; DOI: https://doi.org/10.1007/s11172-021-3075-9.
I. A. Dvornikova, E. V. Buravlev, O. G. Shevchenko, I. Yu. Chukicheva, A. V. Kutchin, Russ. Chem. Bull., 2021, 70, 2185; DOI: https://doi.org/10.1007/s11172-021-3330-0.
E. V. Buravlev, I. Yu. Chukicheva, I. A. Dvornikova, A. V. Churakov, A. V. Kutchin, Russ. J. Org. Chem., 2012, 48, 938; DOI: https://doi.org/10.1134/S1070428012070081.
A. J. Gordon, R. A. Ford. The Chemist’s Companion, New York, John Wiley and Sons, 1972, 560 pp.
F. Yu. Rachinskiy, M. F. Rachinskaya, Tekhnika laboratornykh rabot [Techniques of Laboratory Work], Khimiya, Leningrad, 1982, 432 pp. (in Russian).
M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, G. A. Petersson, H. Nakatsuji, X. Li, M. Caricato, A. V. Marenich, J. Bloino, B. G. Janesko, R. Gomperts, B. Mennucci, H. P. Hratchian, J. V. Ortiz, A. F. Izmaylov, J. L. Sonnenberg, D. Williams-Young, F. Ding, F. Lipparini, F. Egidi, J. Goings, B. Peng, A. Petrone, T. Henderson, D. Ranasinghe, V. G. Zakrzewski, J. Gao, N. Rega, G. Zheng, W. Liang, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, K. Throssell, J. A. Montgomery, Jr., J. E. Peralta, F. Ogliaro, M. J. Bearpark, J. J. Heyd, E. N. Brothers, K. N. Kudin, V. N. Staroverov, T. A. Keith, R. Kobayashi, J. Normand, K. Raghavachari, A. P. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, J. M. Millam, M. Klene, C. Adamo, R. Cammi, J. W. Ochterski, R. L. Martin, K. Morokuma, O. Farkas, J. B. Foresman, D. J. Fox, Gaussian 09 Rev. C.01, Wallingford, CT, 2009.
A. D. Becke, J. Chem. Phys., 1993, 98, 5648; DOI: https://doi.org/10.1063/1.464913.
C. Lee, W. Yang, R. G. Parr, Phys. Rev. B, 1988, 37, 785; DOI: https://doi.org/10.1103/PhysRevB.37.785.
A. J. H. Wachters, J. Chem. Phys., 1970, 52, 1033; DOI: https://doi.org/10.1063/1.1673095.
A. D. McLean, G. S. Chandler, J. Chem. Phys., 1980, 72, 5639; DOI: https://doi.org/10.1063/1.438980.
S. Grimme, J. Antony, S. Ehrlich, H. Krieg, J. Chem. Phys., 2010, 132, 154104; DOI: https://doi.org/10.1063/1.3382344.
J. Tomasi, B. Mennucci, R. Cammi, Chem. Rev., 2005, 105, 2999; DOI: https://doi.org/10.1021/cr9904009.
J. R. Cheeseman, G. W. Trucks, T. A. Keith, M. J. Frisch, J. Chem. Phys., 1996, 104, 5497; DOI: https://doi.org/10.1063/1.471789.
L. Valgimigli, K. U. Ingold, J. J. Lusztyk, J. Org. Chem., 1996, 61, 7947; DOI: https://doi.org/10.1021/jo9608578.
L. Valgimigli, J. T. Banks, J. Lusztyk, K. U. Ingold, J. Org. Chem., 1999, 64, 3383; DOI: https://doi.org/10.1021/jo982360z.
G. Litwinenko, E. Megie, M. Wojnicz, Org. Lett., 2002, 4, 2425; DOI: https://doi.org/10.1021/ol0261837.
I. Wawer, Z. Kecki, Berichte der Bunsen-Gesellschaft, 1976, 80, 522; DOI: https://doi.org/10.1002/bbpc.19760800612.
G. R. Wiley, S. I. Miller, J. Am. Chem. Soc., 1972, 94, 3287; DOI: https://doi.org/10.1021/ja00765a001.
L. J. Bellamy, G. Eglinton, J. F. Morman, J. Chem. Soc., 1961, 4762; DOI: https://doi.org/10.1039/jr9610004762.
C. Reichard, Solvents and Solvent Effects in Organic Chemistry, Verlag Chemie, Weinheim, 1988, 534 pp.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Dedicated to the Academician of the Russian Academy of Sciences V. A. Tartakovsky on the occasion of his 90th birthday.
This work was were carried out within the framework of the State Assignment to the Ufa Institute of Chemistry, Ufa Federal Research Centre of the Russian Academy of Sciences (Project No. 122031400282-9) and to the Institute of Chemistry, Komi Science Center, Ural Branch of the Russian Academy of Sciences (Project No. 1021062211116-4-1.4.1).
No human or animal subjects were used in this research.
The authors declare no competing interests.
Published in Russian in Izvestiya Akademii Nauk. Seriya Khimicheskaya, No. 9, pp. 1856–1862, September, 2022.
Rights and permissions
About this article
Cite this article
Sadykov, R.A., Safina, G.D., Khursan, S.L. et al. Thermodynamic parameters of complexation of sterically hindered phenols with hydrogen bond acceptor solvents: determination by 1H NMR spectroscopy. Russ Chem Bull 71, 1856–1862 (2022). https://doi.org/10.1007/s11172-022-3602-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11172-022-3602-3