Abstract
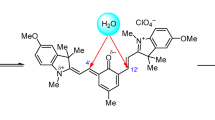
The synthesis of new indoline spiropyran by the cyclocondensation in methanol revealed that the free aldehyde group of the target spiropyran is easily converted into the dimethyl acetal moiety without the addition of an acid catalyst, giving 8′-dimethoxymethyl-1,3,3-trimethylspiro[indoline-2,2′-2H-chromene]-5,6′-dicarboxylic acid as a single reaction product. The structure of this compound was confirmed by NMR spectroscopy and mass spectrometry. The molecular structure was also established by single-crystal X-ray diffraction. The Hirshfeld surfaces were generated and analyzed and intermolecular interactions in the crystal were investigated using the CrystalExplorer software package. The reverse hydrolysis reaction of dimethyl acetal to the aldehyde group proceeds under mild conditions in dimethyl sulfoxide, as shown by NMR spectroscopy.
Similar content being viewed by others
References
H. Bouas-Laurent, H. Dürr, Pure Appl. Chem., 2001, 73, 639; DOI: https://doi.org/10.1351/pac200173040639.
V. X. Truong, K. Ehrmann, M. Seifermann, P. A. Levkin, C. Barner-Kowollik, Chem. Eur. J., 2022, e202104466; DOI: https://doi.org/10.1002/chem.202104466.
W. Szymanski, J. M. Beierle, H. A. Kistemaker, W. A. Velema, B. L. Feringa, Chem. Rev., 2013, 113, 6114; DOI: https://doi.org/10.1021/cr300179f.
R. C. Bertelson, in Organic Photochromic and Thermochromic Compounds. Topics in Applied Chemistry, Eds J. C. Crano, R. J. Guglielmetti, Springer, Boston, MA, 2002, p. 11; DOI: https://doi.org/10.1007/0-306-46911-1_2.
S. Aldoshin, in Organic Photochromic and Thermochromic Compounds. Topics in Applied Chemistry, Eds J. C. Crano, R. J. Guglielmetti, Springer, Boston, MA, 2002, p. 297; DOI: https://doi.org/10.1007/0-306-46912-X_8.
A. K. Chibisov, H. Görner, J. Phys. Chem. A, 1997, 101, 4305; DOI: https://doi.org/10.1021/jp9625691.
A. D. Pugachev, I. V. Ozhogin, M. B. Lukyanova, B. S. Lukyanov, A. S. Kozlenko, I. A. Rostovtseva, N. I. Makarova, V. V. Tkachev, S. M. Aldoshin, A. V. Metelitsa, J. Mol. Struct., 2021, 1229, 129615; DOI: https://doi.org/10.1016/.molstruc.2020.129615.
A. S. Kozlenko, N. I. Makarova, I. V. Ozhogin, A. D. Pugachev, M. B. Lukyanova, I. A. Rostovtseva, G. S. Borodkin, N. V. Stankevich, A. V. Metelitsa, B. S. Lukyanov, Mendeleev Commun., 2021, 31, 403; DOI: https://doi.org/10.1016/j.mencom.2021.05.040.
I. V. Ozhogin, V. V. Chernyavina, B. S. Lukyanov, V. I. Malay, I. A. Rostovtseva, N. I. Makarova, V. V. Tkachev, M. B. Lukyanova, A. V. Metelitsa, S. M. Aldoshin, J. Mol. Struct., 2019, 1196, 409; DOI: https://doi.org/10.1016/j.molstruc.2019.06.094.
R. Klajn, Chem. Soc. Rev., 2014, 43, 148; DOI: https://doi.org/10.1039/C3CS60181A.
A. D. Pugachev, E. L. Mukhanov, I. V. Ozhogin, A. S. Kozlenko, A. V. Metelitsa, B. S. Lukyanov, Chem. Heterocycl. Compd., 2021, 57, 122; DOI: https://doi.org/10.1007/s10593-021-02881-y.
I. V. Ozhogin, P. V. Zolotukhin, V. V. Tkachev, A. D. Pugachev, A. S. Kozlenko, A. A. Belanova, S. M. Aldoshin, B. S. Lukyanov, Russ. Chem. Bull., 2021, 70, 1388; DOI: https://doi.org/10.1007/s11172-021-3228-x.
P. Liu, X. Li, H. Zhang, W. Li, S. Li, Y. Ren., H. Shi, X Li, Prog. Org. Coat., 2022, 156, 106259; DOI: https://doi.org/10.1016/j.porgcoat.2021.106259.
A. Fagan, M. Bartkowski, S. Giordani, Front. Chem., 2021, 612; DOI: https://doi.org/10.3389/fchem.2022.859450.
S. Heng, X. Zhang, J. Pei, A. Adwal, P. Reineck, B. C. Gibson, M. R. Hutchinson, A. D. Abell, Eur. J. Chem., 2019, 25, 854; DOI: https://doi.org/10.1002/chem.201804816.
D. B. Stubing, S. Heng, A. D. Abell, Org. Biomol. Chem., 2016, 14, 3752; DOI: https://doi.org/10.1039/C6OB00468G.
S. Heng, X. Zhang, J. Pei, A. D. Albell, Biosensors, 2017, 7, 36; DOI: https://doi.org/10.3390/bios7030036.
S. Wan, Y. Zheng, J. Shen, W. Yang, M. Yin, ACS Appl. Mater. Interfaces, 2014, 6, 19515; DOI: https://doi.org/10.1021/am506641t.
M. J. Ferronato, D. J. Obiol, E. N. Alonso, J. A. Guevara, S. M. Grioli, M. Mascaro, M. L. Rivadulla, A. Martinez, G. Gomez, Y. Fall, M. A. Quevedo, A. C. Curino, M. M. Facchinetti, J. Steroid Biochem. Mol., 2019, 185, 185; DOI: https://doi.org/10.1016/j.jsbmb.2018.08.006.
D. Dattler, G. Fuks, J. Heiser, E. Moulin, A. Perrot, X. Yao, Chem. Rev., 2020, 120, 310; DOI: https://doi.org/10.1021/acs.chemrev.9b00288.
A. S. Kozlenko, A. D. Pugachev, I. V. Ozhogin, I. M. El-Sewify, B. S. Lukyanov, Chem. Heterocycl. Compd., 2021, 57, 984; DOI: https://doi.org/10.1007/s10593-021-03010-5.
A. Vasilev, L. Engman, J. Org. Chem., 2000, 65, 2151; DOI: https://doi.org/10.1021/jo9917644.
Z. Wu, Q. Wang, P. Li, B. Fang, M. Yin, J. Mater. Chem. C, 2021, 9, 6290; DOI: https://doi.org/10.1039/D1TC00974E.
C. Jelsch, K. Ejsmontb, L. Hudera, IUCrJ., 2014, 1, 119; DOI:10.1107/S2052252514003327.
M. A. Spackman, D. Jayatilaka, CrystEngComm., 2009, 11, 19; DOI: https://doi.org/10.1039/B818330A.
A. D. Pugachev, V. V. Tkachev, S. M. Aldoshin, N. I. Makarova, I. A. Rostovtseva, A. V. Metelitsa, N. V. Stankevich, G. V. Shilov, B. S. Lukyanov, Russ. J. Gen. Chem., 2021, 91, 1297; DOI: https://doi.org/10.1134/S1070363221070069.
A. D. Pugachev, V. V. Tkachev, I. V. Ozhogin, M. B. Lukyanova, S. M. Aldoshin, V. I. Minkin, E. L. Mukhanov, A. V. Metelitsa, N. V. Stankevich, B. S. Lukyanov, Russ. Chem. Bull., 2021, 70, 2090; DOI: https://doi.org/10.1007/s11172-021-3320-2.
N. E. Gel’man, E. A. Terent’eva, T. M. Shanina, L. M. Kiparenko, Metody kolichestvennogo organicheskogo elementnogo analiza [Methods of Quantitative Organic Elemental Analysis], Khimiya, Moscow, 1987, 296 pp. (in Russian).
Y. Kadoya, M. Hata, Y. Tanaka, A. Hirohata, Y. Hitomi, M. Kodera, Inorg. Chem., 2021, 60, 5474; DOI: https://doi.org/10.1021/acs.inorgchem.0c02954.
G. M. Sheldrick, SHELXTL v. 6.14, Structure Determination Software Suite, Bruker AXS, Madison, Wisconsin (USA), 2000.
P. R. Spackman, M. J. Turner, J. J. McKinnon, S. K. Wolff, D. J. Grimwood, D. Jayatilaka, M. A. Spackman, J. Appl. Cryst., 2021, 54, 1006; DOI: https://doi.org/10.1107/S1600576721002910.
C. F. Macrae, I. Sovago, S. J. Cottrell, P. T. A. Galek, P. McCabe, E. Pidcock, M. Platings, G. P. Shields, J. S. Stevens, M. Towler, P. A. Wood, J. Appl. Cryst., 2020, 53, 226; DOI: https://doi.org/10.1107/S1600576719014092.
Author information
Authors and Affiliations
Corresponding author
Additional information
Dedicated to Academician of the Russian Academy of Sciences V. A. Tartakovsky on the occasion of his 90th birthday.
This work was carried out with the financial support from the Russian Science Foundation (Project No. 21-73-10300, https://rscf.ru/project/21-73-10300/) in the Southern Federal University. The X-ray diffraction analysis was performed by V. V. Tkachev, G. V. Shilov, and S. M. Aldoshin within the framework of the state assignment to the Institute of Problems of Chemical Physics of the Russian Academy of Sciences (No. AAAA-A19-119092390076-7).
No human or animal subjects were used in this research.
The authors declare no competing interests.
Published in Russian in Izvestiya Akademii Nauk. Seriya Khimicheskaya, No. 8, pp. 1710–1719, August, 2022.
Rights and permissions
About this article
Cite this article
Ozhogin, I.V., Pugachev, A.D., Tkachev, V.V. et al. Synthesis and study of interconversions of new indoline spiropyrans based on 4-hydroxy-3,5-diformylbenzoic acid. Russ Chem Bull 71, 1710–1719 (2022). https://doi.org/10.1007/s11172-022-3581-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11172-022-3581-4