Abstract
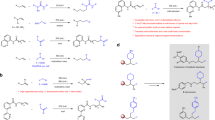
Various methods for the reduction of (het)arylimines of cage-structured monoterpenoid ketones, l-fenchone and d-camphor, were explored. The best results were achieved using the NaBH4-NiCl2 • 6H2O system in 95% ethanol. The exception is halogenated aniline derivatives, in the case of which the reduction of the C-N bond is accompanied with the hydrogenolysis of the C-Hal bond.
Similar content being viewed by others
References
A. S. Sokolova, O. I. Yarovaya, D. I. Korchagina, V. V. Zarubaev, T. S. Tretiak, P. M. Anfimov, O. I. Kiselev, N. F. Salakhutdinov, Bioorg. Med. Chem., 2014, 22, 2141; DOI: https://doi.org/10.1016/j.bmc.2014.02.038.
Pat. FR 1302373; 1962.
Pat. US 6720338; 2004.
I. S. Morozov, V. I. Petrov, S. A. Sergeeva, Farmakologia adamantanov [Pharmacology of Adamantanes], Volgograd Medical Academy, Volgograd, 2001, p. 320 (in Russian).
R. Leiva, S. Vázquez, M. Barniol-Xicota, S. Codony, T. Ginex, E. Vanderlinden, M. Montes, M. Caffrey, F. J. Luque, L. Naesens, S. Vázquez, J. Med. Chem., 2018, 61, 98; DOI: https://doi.org/10.1021/acs.jmedchem.7b00908.
S. N. Tandura, V. V. Zarubayev, P. M. Anfimov, O. I. Kiselev, Antibiotiki i khimioterapia [Antibiotics and Chemotherapy], 2013, 58, No. 1–2, 36 (in Russian).
Pat. US 2211629; 1936.
A. A. Vernigora, D. A. Nilidin, A. V. Davidenko, Phan Ngok Tu, S. G. Gubin, E. V. Gubina, M. A. Vaniev, I. A. Novakov, Izv. VolgGTU [Proc. of Volg. St. T. Univ.], 2021, 252, 47 (in Russian); DOI: https://doi.org/10.35211/1990-5297-2021-5-252-47-52.
J. A. Gardner, G. B. Cockburn, J. Chem. Soc., 1898, 73, 275; DOI: https://doi.org/10.1039/CT8987300275.
O. Wallach, Justus Liebigs Ann. Chem., 1891, 263, 129; DOI: https://doi.org/10.1002/jlac.18912630202.
O. Wallach, P. Vivck, Justus Liebigs Ann. Chem., 1908, 362, 174; DOI: https://doi.org/10.1002/jlac.19083620203.
Y. Suzuki, Y. Ogata, K. Hiroi, Tetrahedron: Asymmetry, 1999, 10, 1219; DOI: https://doi.org/10.1016/S0957-4166(99)00108-1.
K. Hiroi, K. Watanabe, Tetrahedron: Asymmetry, 2001, 12, 3067; DOI: https://doi.org/10.1016/S0957-4166(01)00546-8.
Pat. RU 2751773; 2021.
M. Periasamy, A. Devasagayaraj, N. Satyanarayana, C. Narayana, Synth. Commun., 1989, 19, 565; DOI: https://doi.org/10.1080/00397918908050701.
N. M. Yoon, H. C. Brown, J. Am. Chem. Soc., 1968, 90, 2927; DOI: https://doi.org/10.1021/ja01013a033.
A. S. B. Prasad, J. V. B. Kanth, M. Periasamy, Tetrahedron, 1992, 48, 4623; DOI: https://doi.org/10.1016/S0040-4020(01)81236-9.
D. Papa, E. Schwenk, B. Whitman, J. Org. Chem., 1942, 7, 587; DOI: https://doi.org/10.1021/jo01200a017.
I. A. Novakov, B. S. Orlinson, R. V. Brunilin, M. B. Navrotskii, A. S. Eremiichuk, S. A. Dumler, E. A. Gordeeva, Pharm. Chem. J. (Engl.Transl.), 2011, 45, 419; DOI: https://doi.org/10.1007/s11094-011-0646-3.
Pat. RU 2307826; 2007.
Pat. RU 2750161; 2021.
A. S. Sokolova, O. I. Yarovaya, N. I. Bormotov, L. N. Shishkina, N. F. Salakhutdinov, Chem. Biodiversity, 2018, 15, e1800153; DOI: https://doi.org/10.1002/cbdv.201800153.
L. F. Tietze, T. Eicher, Reaktionen und Synthesen im organisch-chemischen. Praktikum und Forschungslaboratorium, 2. Aufl., Georg Thieme Verlag, Stuttgart—New York, 1991, p. 672.
P. Lipp, G. Stutzinger, Chem. Ber., 1932, 65, 241; DOI: https://doi.org/10.1002/cber.19320650227.
T. Kuwata, Kogyo Kagaku Zasshi [Journal of the Society of Chemical Industry, Japan], 1934, 37, 389; DOI: https://doi.org/10.1246/nikkashi1898.37.supplement_299b.
F. Ullmann, A. Schmid, Chem. Ber., 1910, 43, 3204.
Author information
Authors and Affiliations
Corresponding author
Additional information
Dedicated to Academician of the Russian Academy of Sciences V. A. Tartakovskii on the occasion of his 90th birthday.
This work was financially supported by the Russian Science Foundation (Project No. 22-13-20062, https://rscf.ru/project/22-13-20062/) and by the Administration of the Volgograd Region of the Russian Federation (Agreement No. 2 on June 10, 2022) and performed using the analytical equipment of the Center for Collective Use (CCU) at the N. N. Vorozhtsov Novosibirsk Institute of Organic Chemistry of the Siberian Branch of the Russian Academy of Sciences.
No human or animal subjects were used in this research.
The authors declare no competing interests.
Published in Russian in Izvestiya Akademii Nauk. Seriya Khimicheskaya, No. 8, pp. 1662–1669, August, 2022.
Rights and permissions
About this article
Cite this article
Brunilin, R.V., Vernigora, A.A., Vostrikova, O.V. et al. Development and competitive evaluation of methods for the reduction of (het)arylimines of cage-structured monoterpenoid ketones. Russ Chem Bull 71, 1662–1669 (2022). https://doi.org/10.1007/s11172-022-3576-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11172-022-3576-1