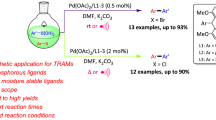
Abstract
Palladium-catalyzed Suzuki cross-coupling of isomeric dichloroacetophenones with phenylboronic acid in the presence of 2-dicyclohexylphosphino-2′-(dimethylamino)-biphenyl (DavePHOS, a Buchwald ligand) affords the corresponding diphenylacetophenones in high yields. The reaction of pentachloroacetophenone with phenylboronic acid (1.1–2.5 equiv.) proceeds unselectively to give isomeric mono- and disubstitution products. In the case of 3,4-dichloroanisole, 3-positioned chlorine atom is preferably replaced, however, with excess phenylboronic acid 4′;-methoxy-1,1′;:2′;,1″-terphenyl is quantitatively formed.
Similar content being viewed by others
References
J. P. Wolfe, R. A. Singer, B. H. Yang, S. L. Buchwald, J. Am. Chem. Soc., 1999, 121, 9550; DOI: https://doi.org/10.1021/ja992130h.
T. E. Barder, S. D. Walker, J. R. Martinelli, S. L. Buchwald, J. Am. Chem. Soc., 2005, 127, 4685; DOI: https://doi.org/10.1021/ja042491j.
R. Martin, S. Buchwald, Acc. Chem. Res., 2008, 41, 1461; DOI: https://doi.org/10.1021/ar800036s.
S. Reimann, P. Ehlers, M. Sharif, A. Spannenberg, P. Langer, Tetrahedron, 2016, 72, 1083; DOI: https://doi.org/10.1016/j.tet.2016.01.010.
D.-H. Lee, M.-J. Jin, Org. Lett., 2011, 13, 252; DOI: https://doi.org/10.1021/o1102677r.
T. Tu, Zh. Sun, W. Fang, M. Xu, Yu. Zhou, Org. Lett., 2012, 14, 4250; DOI: https://doi.org/10.1021/o13019665.
Polychloroaromatic Compounds, Ed. H. Suschitzky, Plenum Press, New York, 1974, 543 pp.
A. A. Vasil’ev, A. S. Burukin, S. G. Zlotin, Russ. Chem. Rev., 2007, 76, 885; DOI: https://doi.org/10.1070/RC2007v076n10ABEH003724.
T. I. Gorbunova, V. I. Saloutin, O. N. Chupakhin, Russ. Chem. Rev., 2010, 79, 511; DOI: https://doi.org/10.1070/RC2010v079n06ABEH004047.
A. A. Vasil’ev, A. S. Burukin, G. M. Zhdankina, S. G. Zlotin, Mendeleev Commun., 2021, 31, 400; DOI: https://doi.org/10.1016/j.mencom.2021.04.039.
A. A. Vasil’ev, A. S. Burukin, G. M. Zhdankina, S. G. Zlotin, Russ. Chem. Bull., 2022, 71, 169; DOI: https://doi.org/10.1007/s11172-022-3392-7.
H. Sajiki, A. Kume, K. Hattori, K. Hirota, Tetrahedron Lett., 2002, 43, 7247; DOI: https://doi.org/10.1016/S0040-4039(02)01622-2.
S. B. Soliev, A. V. Astakhov, D. V. Pasyukov, V. M. Chernyshev, Russ. Chem. Bull., 2020, 69, 683; DOI: https://doi.org/10.1007/s11172-020-2818-3.
N. A. Bumagin, Russ. Chem. Bull., 2021, 70, 1483; DOI: https://doi.org/10.1007/s11172-021-3243-y.
S. A. Rzhevskiy, M. A.Topchiy, V. N. Bogachev, L. I. Minaeva, I. R. Cherkashchenko, K. V. Lavrov, G. K. Sterligov, M. S.Nechaev, A. F. Asachenko, Mendeleev Commun., 2021, 31, 409; DOI: https://doi.org/10.1016/j.mencom.2021.04.042.
V. V. Chesnokov, M. A. Shevchenko, S. B. Soliev, V. A. Tafeenko, V. M. Chernyshev, Russ. Chem. Bull., 2021, 70, 1281; DOI: https://doi.org/10.1007/s11172-021-3212-5.
E. V. Verbitskiy, E. M. Dinastiya, O. S. Eltsov, E. F. Zhilina, A. V. Schepochkin, G. L. Rusinov, O. N. Chupakhin, V. N. Charushin, Mendeleev Commun., 2020, 30, 142; DOI: https://doi.org/10.1016/j.mencom.2020.03.003.
E. S. Matyugina, A. L. Khandazhinskaya, S. N. Kochetkov, K. L. Seley-Radtke, Mendeleev Commun., 2020, 30, 231; DOI: https://doi.org/10.1016/j.mencom.2020.03.034.
L. Cieslakowa, R. Malinowski, B. Sledzinski, Rocz. Chem., 1970, 44, 77.
W. Zielinski, A. Skibinski, Rocz. Chem., 1975, 49, 143.
G. Lock, E. Böck, Ber. Dtsch. Chem. Ges. B, 1937, 70, 916; DOI: https://doi.org/10.1002/cber.19370700509.
A. S. Burukin, A. A. Vasil’ev, M. I. Struchkova, V. V. Kachala, S. G. Zlotin, Russ. Chem. Bull., 2005, 54, 964; DOI: https://doi.org/10.1007/s11172-005-0341-1.
I. V. Kuchurov, A. A. Vasil’ev, S. G. Zlotin, Mendeleev Commun., 2010, 20, 140; DOI: https://doi.org/10.1016/j.mencom.2010.05.005.
T. J. Barton, W. F. Goure, J. L. Witiak, W. D. Wulff, J. Organomet. Chem., 1982, 225, 87; DOI: https://doi.org/10.1016/S0022-328X(00)86813-8.
F. Kakiuchi, S. Kan, K. Igi, N. Chatani, S. Murai, J. Am. Chem. Soc., 2003, 125, 1698; DOI: https://doi.org/10.1021/ja029273f.
J. D. Kehlbeck, E. J. Dimise, S. M. Sparks, S. Ferrara, J. M. Tanski, C. M. Anderson, Synthesis, 2007, 1979; DOI: https://doi.org/10.1055/s-2007-983722.
Author information
Authors and Affiliations
Corresponding author
Additional information
Dedicated to Academician of the Russian Academy of Sciences V. A. Tartakovsky on the occasion of his 90th birthday.
No human or animal subjects were used in this research.
The authors declare no competing interests.
Published in Russian in Izvestiya Akademii Nauk. Seriya Khimicheskaya, No. 8, pp. 1656–1661, August, 2022.
Rights and permissions
About this article
Cite this article
Vasil’ev, A.A., Burukin, A.S., Zhdankina, G.M. et al. Functionalized polychloroarenes in the Bichwald ligand-promoted Suzuki cross-coupling. Russ Chem Bull 71, 1656–1661 (2022). https://doi.org/10.1007/s11172-022-3575-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11172-022-3575-2