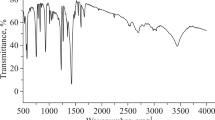
Abstract
Two new compounds, trichloro[O-bis(2-hydroxyethyl)ammonium] manganese(ii), {Mn[(HOCH2CH2)2NH2]Cl3}n (1), and N-(2-hydroxyethyl)-N-methylammonium aquatrichloromanganate(II), [CH3NH2CH2CH2OH][Mn(H2O)Cl3] (2), were synthesized. The crystal structures of the new compounds were determined by single-crystal X-ray diffraction. Both structures contain similar chains composed of (MnOCl5) octahedra. In the structure of 1, the manganese atom is coordinated by the OH group of the cation; in the structure of 2, the manganese atom is coordinated by a water molecule. There are different hydrogen bond systems in the structure of 1 due to disorder of OH groups of the cation. The melting points and enthalpies of the synthesized compounds evaluated by DSC are 162 °C and 17.3 kJ mol−1 for compound 1 and 30 °C and 16.9 kJ mol−1 for compound 2, respectively. Compound 1 is a chain coordination polymer, while compound 2 is an ionic liquid with a polynuclear anion.
Similar content being viewed by others
References
S. Walha, H. Naili, S. Yahyaoui, B. F. Ali, M. M. Turnbull, T. Mhiri, T. Bataille, Sol. State Sci., 2011, 13, 204; DOI: https://doi.org/10.1016/j.solidstatesciences.2010.11.015.
L. J. De Jongh, A. R. Miedema, Adv. Phys., 2001, 50, 947; DOI: https://doi.org/10.1080/00018730110101412.
M.-E. Sun, Y. Li, X.-Y. Dong, S.-Q. Zang, Chem. Sci., 2019, 10, 3836; DOI: https://doi.org/10.1039/c8sc04711a.
B. Su, G. Zhou, J. Huang, E. Song, A. Nag, Z. Xia, Laser Photonics Rev., 2020, 2000334; DOI: https://doi.org/10.1002/lpor.202000334.
B. Luo, F. Li, K. Xu, Y. Guo, Y. Liu, Z. Xia, J. Z. Zhang, J. Mater. Chem. C, 2019, 7, 2781; DOI: https://doi.org/10.1039/C8TC05741A.
J. Estager, J. D. Holbrey, M. Swadźba-Kwaśny, Chem. Soc. Rev., 2014, 43, 847; DOI: https://doi.org/10.1039/C3CS60310E.
A. Zazybin, Kh. Rafikova, V. Yu, D. Zolotareva, V. M. Dembitsky, T. Sasaki, Russ. Chem. Rev., 2017, 86, 1254; DOI: https://doi.org/10.1070/RCR4743.
C. Jiang, H. Fu, Y. Han, D. Li, H. Lin, B. Li, X. Meng, H. Peng, J. Chu, Cryst. Res. Technol., 2019, 54, 1800236; DOI: https://doi.org/10.1002/crat.201800236.
H. Zhang, P. Chen, L. Fang, Acta Cryst., 2006, E62, m658; DOI: https://doi.org/10.1107/S1600536806006751.
X.-F. Sun, P.-F. Li, W.-Q. Liao, Z. Wang, J. Gao, H.-Y. Ye, Y. Zhang, Inorg.Chem., 2017, 56, 12193; DOI: https://doi.org/10.1021/acs.inorgchem.7b01553.
W. Depmeier, Acta Cryst., 1977, B33, 3713; DOI: https://doi.org/10.1107/S0567740877011911.
M. A. Zakharov, Yu. V. Filatova, M. A. Bykov, N. V. Avramenko, L. A. Aslanov, Russ. J. Coord. Chem., 2020, 46, 268; DOI: https://doi.org/10.1134/S1070328420040077.
G. M. Sheldrick, Acta Cryst., 2015, C71, 3; DOI: https://doi.org/10.1107/S2053229614024218.
L. J. Farrugia, J. Appl. Cryst., 1999, 32, 837; DOI: https://doi.org/10.1107/S0021889899006020.
Diamond: Crystal and Molecular Structure Visualization, Bonn, Crystal Impact, 2014.
Funding
This work was financially supported by the Russian Foundation for Basic Research (Project No. 19-08-00672a).
Author information
Authors and Affiliations
Corresponding author
Additional information
No human or animal subjects were used in this research.
The authors declare no competing interests.
Based on the materials of the XXVIII International Chugaev Conference on Coordination Chemistry (October 3–8, 2021, Tuapse).
Published in Russian in Izvestiya Akademii Nauk. Seriya Khimicheskaya, No. 6, pp. 1240–1246, June, 2022.
Rights and permissions
About this article
Cite this article
Zakharov, M.A., Filatova, Y.V., Mikheeva, S.R. et al. Synthesis, crystal structures, and thermal properties of trichloro[O-bis(2-hydroxyethyl)ammonium] manganese(ii), {Mn[(HOCH2CH2)2NH2]Cl3}n, and N-(2-hydroxyethyl)-N-methylaminoniuin aquatrichloromanganate(ii), [MeNH2CH2CH2OH][Mn(H2O)Cl3]. Russ Chem Bull 71, 1240–1246 (2022). https://doi.org/10.1007/s11172-022-3525-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11172-022-3525-z