Abstract
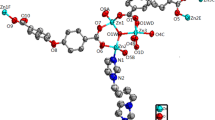
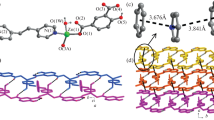
A three-dimensional coordination polymer was synthesized by the reaction of zinc(ii) nitrate with 4,7-(4-carboxyphenyl)-2,1,3-benzoxadiazole and di(imidazol-1-yl)methane. The crystal structure of the new compound was determined by single-crystal X-ray diffraction. In the structure of the metal-organic framework, the trinuclear secondary building units are connected to each other to form a uninodal eight-connected net with the symbol {33.418.55.62}. This topology has not been previously found in coordination polymers. The framework contains channels with dimensions of 7×8 Å2. The solvent-accessible volume is 24%. The compound exhibits emission with a maximum at 524 nm upon excitation at 395 nm. The photoluminescence quantum yield is 19%.
Similar content being viewed by others
References
S.-N. Zhao, G. Wang, D. Poelman, P. Voort, S.-N. Zhao, G. Wang, D. Poelman, P. Van Der Voort, Materials, 2018, 11, 572; DOI: https://doi.org/10.3390/ma11040572.
S. L. Jackson, A. Rananaware, C. Rix, S. V. Bhosale, K. Latham, Cryst. Growth Des., 2016, 16, 3067; DOI:. https://doi.org/10.1021/acs.cgd.6b00428.
A. Kuznetsova, V. Matveevskaya, D. Pavlov, A. Yakunenkov, A. Potapov, Materials, 2020, 13, 2699; DOI: https://doi.org/10.3390/ma13122699.
T. S. Sukhikh, D. S. Ogienko, D. A. Bashirov, S. N. Konchenko, Russ. Chem. Bull., 2019, 68, 651; DOI: https://doi.org/10.1007/s11172-019-2472-9.
B. A. D. Neto, A. A. M. Lapis, E. N. Da Silva Júnior, J. Dupont, Eur. J. Org. Chem., 2013, 228; DOI: https://doi.org/10.1002/ejoc.201201161.
T. S. Sukhikh, R. M. Khisamov, D. A. Bashirov, V. Y. Komarov, M. S. Molokeev, A. A. Ryadun, E. Benassi, S. N. Konchenko, Cryst. Growth Des., 2020, 20, 5796; DOI: https://doi.org/10.1021/acs.cgd.0c00406.
R. J. Marshall, Y. Kalinovskyy, S. L. Griffin, C. Wilson, B. A. Blight, R. S. Forgan, J. Am. Chem. Soc., 2017, 139, 6253; DOI: https://doi.org/10.1021/jacs.7b02184.
A. Mallick, A. M. El-Zohry, O. Shekhah, J. Yin, J. Jia, H. Aggarwal, A.-H. Emwas, O. F. Mohammed, M. Eddaoudi, J. Am. Chem. Soc., 2019, 141, 7245; DOI: https://doi.org/10.1021/jacs.9b01839.
X. Luo, L. Kan, X. Li, L. Sun, G. Li, J. Zhao, D.-S. Li, Q. Huo, Y. Liu, Cryst. Growth Des., 2016, 16, 7301; DOI: https://doi.org/10.1021/acs.cgd.6b01539.
X. Han, Q. Cheng, X. Meng, Z. Shao, K. Ma, D. Wei, J. Ding, H. Hou, Chem. Commun., 2017, 53, 10314; DOI: https://doi.org/10.1039/C7CC06125K.
Z. Ju, W. Yan, X. Gao, Z. Shi, T. Wang, H. Zheng, Cryst. Growth Des., 2016, 16, 2496; DOI: https://doi.org/10.1021/acs.cgd.5b00681.
C. Song, Y. Ling, L. Jin, M. Zhang, D.-L. Chen, Y. He, Dalt. Trans., 2016, 45, 190; DOI: https://doi.org/10.1039/C5DT02845K.
S. Wu, D. Ren, K. Zhou, H.-L. Xia, X.-Y. Liu, X. Wang, J. Li, J. Am. Chem. Soc., 2021, 143, 10547; DOI: https://doi.org/10.1021/jacs.1c04810.
V. A. Blatov, A. P. Shevchenko, D. M. Proserpio, Cryst. Growth Des., 2014, 14, 3576; DOI: https://doi.org/10.1021/cg500498k.
C. Bonneau, M. O’Keeffe, D. M. Proserpio, V. A. Blatov, S. R. Batten, S. A. Bourne, M. S. Lah, J.-G. Eon, S. T. Hyde, S. B. Wiggin, L. Öhrström, Cryst. Growth Des., 2018, 18, 3411; DOI: https://doi.org/10.1021/acs.cgd.8b00126.
A. R. C. Hinojosa, S. P. de Souza, T. V. Alves, I. T. O. dos Santos, E. O. Silva, I. L. Gonçalves, A. A. Merlo, C. F. Junkes, I. H. Bechtold, A. A. Vieira, J. Mol. Liq., 2021, 338, 116614; DOI: https://doi.org/10.1016/j.molliq.2021.116614.
V. M. Korshunov, M. S. Mikhailov, T. N. Chmovzh, A. A. Vashchenko, N. S. Gudim, L. V Mikhalchenko, I. V. Taydakov, O. A. Rakitin, Molecules, 2021, 26, 2872; DOI: https://doi.org/10.3390/molecules26102872.
V. A. Lazarenko, P. V. Dorovatovskii, Y. V. Zubavichus, A. S. Burlov, Y. V. Koshchienko, V. G. Vlasenko, V. N. Khrustalev, Crystals, 2017, 7, 325; DOI: https://doi.org/10.3390/cryst7110325.
R. D. Svetogorov, P. V. Dorovatovskii, V. A. Lazarenko, Crystal Research and Technology, 2020, 55, 1900184; DOI: https://doi.org/10.1002/crat.201900184.
W. Kabsch, Acta Crystallogr. Sect. D: Biol. Crystallogr., 2010, 66, 125; DOI: https://doi.org/10.1107/S0907444909047337.
W. Kabsch, Acta Crystallogr., Sect. D: Biol. Crystallogr., 2010, 66, 133; DOI: https://doi.org/10.1107/S0907444909047374.
G. M. Sheldrick, Acta Crystallogr. Sect. A., 2015, 71, 3; DOI: https://doi.org/10.1107/S2053273314026370.
G. M. Sheldrick, Acta Crystallogr. Sect. C., 2015, 71, 3; DOI: https://doi.org/10.1107/S2053229614024218.
Author information
Authors and Affiliations
Corresponding author
Additional information
Based on the materials of the All-Russian Congress on Chemistry of Heterocyclic Compounds (October 12–16, 2021, Sochi, Russia).
Published in Russian in Izvestiya Akademii Nauk. Seriya Khimicheskaya, No. 5, pp. 974–979, May, 2022.
This study was financially supported by the Russian Science Foundation (Project No. 19-73-20087). Physicochemical studies of the synthesized compounds were performed at the Center of Shared Use of the A. V. Nikolaev Institute of Inorganic Chemistry, Siberian Branch of the Russian Academy of Sciences, with the financial support from the Ministry of Science and Higher Education of the Russian Federation (Project Nos 121031700321-3 and 121031700313-8).
No human or animal subjects were used in this research.
The authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Pavlov, D.I., Poklonova, V.V., Ryadun, A.A. et al. Synthesis and crystal structure of a luminescent metal-organic framework based on 4,7-(4-carboxyphenyl)-2,1,3-benzoxadiazole. Russ Chem Bull 71, 974–979 (2022). https://doi.org/10.1007/s11172-022-3499-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11172-022-3499-x