Abstract
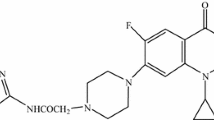
Fluoroquinolone antibiotics (norfloxacin, ciprofloxacin, and moxifloxacin) modified at the piperazine ring by sterically hindered phenolic moieties were synthesized and evaluated for antibacterial activity. It was found that this modification can lead to an increase in the antibacterial activity against Gram-positive strains of the bacteria Staphylococcus aureus ATCC 29213 and St. epidermis compared to parent fluoroquinolones. Modified compounds additionally containing the phosphonate moiety exhibit the highest activity against St. epidermis. Moxifloxacin containing the 3,5-di-tert-butyl-4-hydroxybenzyl moiety is more active against Escherichia coli ATCC 25922 than all the characterized unmodified fluoroquinolones. Ciprofloxacin derivatives bearing sterically hindered phenolic moieties exhibited high activity against clinical isolates of Gram-negative bacteria.
Similar content being viewed by others
References
V. N. Charushin, E. V. Nosova, G. N. Lipunova, O. N. Chupakhin, Ftorkhinolony. Sintez i primenenie [Fluoroquinolones, Synthesis and Application], Fizmatlit, Moscow, 2014, pp. 8–9 (in Russian).
E. V. Nosova, O. A. Batanova, N. N. Mochulskaya, V. N. Charushin, Chem. Heterocycl. Compd., 2019, 55, 578; DOI: https://doi.org/10.1007/s10593-019-02499-1.
Yu. G. Shtyrlin, A. S. Petukhov, A. D. Strelnik, N. V. Shtyrlin, A. G. Iksanova, M. V. Pugachev, R. S. Pavelyev, M. S. Dzyurkevich, M. R. Garipov, K. V. Balakin, Russ. Chem. Bull., 2019, 68, 911; DOI: https://doi.org/10.1007/s11172-019-2504-5.
G.-F. Zhang, X. Liu, S. Zhang, B. Pan, M.-L. Liu, Eur. J. Med. Chem., 2018, 146, 599; DOI: https://doi.org/10.1016/j.ejmech.2018.01.078.
G. N. Lipunova, E. V. Nosova, N. N. Mochul’skaya, L. P. Sidorova, E. V. Tsoi, G. A. Mokrushina, O. M. Chasovskikh, V. N. Charushin, M. A. Kravchenko, Pharm. Chem. J., 2004, 38, 597; DOI: https://doi.org/10.1007/s11094-005-0037-8.
J. C. McPherson III, R. Runner, T. B. Buxton, J. F. Hartmann, D. Farcasiu, I. Bereczki, E. Roth, S. Tollas, E. Ostorházi, F. Rozgonyi, P. Herczegh, Eur. J. Med. Chem., 2012, 47, 615; DOI: https://doi.org/10.1016/j.ejmech.2011.10.049.
T. J. Houghton, K. S. E. Tanaka, T. Kang, E. Dietrich, Y. Lafontaine, D. Delorme, S. S. Ferreira, F. Viens, F. F. Arhin, I. Sarmiento, D. Lehoux, I. Fadhil, K. Laquerre, J. Liu, V. Ostiguy, H. Poirier, G. Moeck, T. R. Parr Jr., A. R. Far, J. Med. Chem., 2008, 51, 6955; DOI: https://doi.org/10.1021/jm801007z.
K. S. E. Tanaka, T. J. Houghton, T. Kang, E. Dietrich, D. Delorme, S. S. Ferreira, L. Caron, F. Viens, F. F. Arhin, I. Sarmiento, D. Lehoux, I. Fadhil, K. Laquerre, J. Liu, V. Ostiguy, H. Poirier, G. Moeck, T.R. Parr Jr., A. R. Far, Bioorg. Med. Chem., 2008, 16, 9217; DOI: https://doi.org/10.1016/j.bmc.2008.09.010.
P. Herczegh, T. B. Buxton, J. C. McPherson III, A. Kovács-Kulyassa, P. D. Brewer, F. Sztaricskai, G. G. Stroebel, K. M. Plowman, D. Farcasin, J. F. Hartmann, J. Med. Chem., 2002, 45, 2338; DOI: https://doi.org/10.1021/jm0105326.
E. B. Menshchikova, V. Z. Lankin, N. V. Kandalintseva, Fenol’nye antioksidanty v biologii i meditsine. Stroenine, svoistva, mekhanizmy deistviya [Phenolic Antioxidants in Biology and Medicine. Structure, Properties, Mechanisms of Action], LAP LAMBERT Academic Publishing, Saarbrücken, 2013, 488 pp. (in Russian).
E. V. Buravlev, O. G. Shevchenko, A. V. Kutchin, Russ. Chem. Bull., 2021, 70, 183; DOI: https://doi.org/10.1007/s11172-021-3075-9.
E. V. Buravlev, I. V. Fedorova, O. G. Shevchenko, A. V. Kutchin, Russ. Chem. Bull., 2020, 69, 1573; DOI: https://doi.org/10.1007/s11172-020-2937-x.
A. V. Bogdanov, E. F. Akhmetova, S. V. Bukharov, V. F. Mironov, Russ. J. Gen. Chem., 2014, 84, 1860; DOI: https://doi.org/10.1134/S1070363214090370.
S. V. Bukharov, G. N. Nugumanova, N. A. Mukmeneva, A. R. Burilov, E. M. Kasymova, M. A. Pudovik, A. I. Konovalov, Russ. J. Org. Chem., 2004, 40, 293.
Yu. N. Oludina, E. D. Ibatullina, S. V. Bukharov, G. N. Nugumanova, R. G. Tagasheva, R. Ya. Deberdeev, Russ. J. Gen. Chem., 2015, 85, 383; DOI: https://doi.org/10.1134/S1070363215020061.
S. V. Bukharov, D. F. Bakhdyrova, R. G. Tagasheva, A. R. Burilov, I. A. Litvinov, D. V. Chachkov, Ya. A. Vereshchagina, Russ. Chem. Bull., 2021, 70, 1964; DOI: https://doi.org/10.1007/s11172-021-3304-2.
Cambrige Structural Database System, Ver. 5.41, 2020.
Y.-H. Li, R.-G. Xiong, Chin. J. Inorg. Chem., 2005, 81, 571.
E. V. Nikitina, M. I. Zeldi, M. V. Pugachev, S. V. Sapozhnikov, N. V. Shtyrlin, S. V. Kuznetsova, V. E. Evtygin, A. R. Kayumov, Y. G. Shtyrlin, M. I. Bogachev, World J. Microbiol. Biotechnol., 2016, 32, Art. № 5, DOI: https://doi.org/10.1007/s11274-015-1969-0.
Pat. RF 2017717, Byul. Izobret. [Inventor Bull.], 1994, No. 15 (in Russian).
S. D. Pastor, P. A. Odorisio, R. Ravichandran, Phosphorus, Sulfur Silicon Relat. Elem., 1987, 29, 67; DOI: https://doi.org/10.1080/03086648708072842.
E. M. Gibadullina, T. R. Shaekhov, Yu. K. Voronina, M. A. Pudovik, A. R. Burilov, Russ. J. Org. Chem., 2018, 54, 530; DOI: https://doi.org/10.1134/S1070428018040036.
G. M. Sheldrick, SADABS, Bruker AXS Inc., Madison, USA, 1997.
G. M. Sheldrick, Acta Crystallogr., Sect. A: Found. Adv., 2015, 71, 3; DOI: https://doi.org/10.1107/S2053273314026370.
G. M. Sheldrick, Acta Crystallogr., Sect. C: Struct. Chem., 2015, 71, 3; DOI: https://doi.org/10.1107/S2053229614024218.
L. J. Farrugia, J. Appl. Crystallogr., 2012, 45, 849; DOI: https://doi.org/10.1107/S0021889812029111.
A. N. Mironov, Rukovodstvo po provedeniyu doklinicheskikh issledovanii lekarstvennykh sredstv. Chast’ pervaya [Manual on Preclinical Studies of Pharmaceuticals. Part 1], Grif i K, Moscow, 2012, 944 pp. (in Russian).
N. V. Shtyrlin, R. M. Vafina, M. V. Pugachev, R. M. Khaziev, E. V. Nikitina, M. I. Zeldi, A. G. Iksanova, Yu. G. Shtyrlin, Russ. Chem. Bull., 2016, 65, 537; DOI: https://doi.org/10.1007/s11172-016-1334-y.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare no competing interests.
Additional information
We are grateful to the staff of the Joint Spectral-Analytical Center for Physico-Chemical Studies of the Structure, Properties and Composition of Substances and Materials of the Federal Research Center “Kazan Scientific Center of Russian Academy of Sciences” for technical support of our studies.
No human or animal subjects were used in this research.
Rights and permissions
About this article
Cite this article
Bukharov, S.V., Tagasheva, R.G., Litvinov, I.A. et al. Synthesis and antibacterial activity of fluoroquinolones with sterically hindered phenolic moieties. Russ Chem Bull 71, 508–516 (2022). https://doi.org/10.1007/s11172-022-3441-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11172-022-3441-2