Abstract
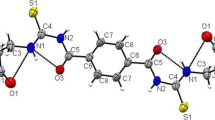
Protolytic and complexing properties of N-[1,1-bis(hydroxymethyl)ethyl]- and N-[tris-(hydroxymethyl)methyl]-β-alanine were estimated using the potentiometry. Values of the dissociation constants of amino and carboxyl functional groups in molecules of these reagents, as well as the stability constants of their complexes of various compositions with copper(ii), cobalt(ii), nickel(ii), zinc(ii), cadmium(ii), silver(i), magnesium(ii), calcium(ii), strontium(ii), and barium(ii) ions, were calculated. The hydroxyalkylation of β-alanine tunes its properties towards the considered metal ions. Complexes of copper(ii) ions with these β-alanine derivatives are the most stable complex compounds among explored herein.
Similar content being viewed by others
References
N. V. Shcheglova, T. V. Popova, Russ. Chem. Bull., 2020, 69, 1771; DOI: https://doi.org/10.1007/s11172-020-2961-x.
N. S. Kiprova, Yu. A. Kondratenko, V. L. Ugolkov, V. V. Gurzhiy, T. A. Kochina, Russ. Chem. Bull., 2020, 69, 1789; DOI: https://doi.org/10.1007/s11172-020-2963-8.
R. N. Roy, L. N. Roy, I. B. Henson, J. M. Stegner, J. J. Dinga, C. E. Summers, L. A. Dieterman, J. Chem. Thermodyn., 2012, 52, 11; DOI: https://doi.org/10.1016/j.jct.2012.02.019.
V. Virtanen, G. Bordin, Anal. Chim. Acta, 1999, 402, 59; DOI: https://doi.org/10.1016/s0003-2670(99)00545-0.
N. Sharma, R. Sharma, Y. S. Rajput, B. Mann, K. Gandhi, Food Chem., 2020, 127923; DOI: https://doi.org/10.1016/j.foodchem.2020.127923.
T. I. Williams, J. C. Combs, A. P Thakur, H. J. Strobel, B. C. Lynn, Electrophoresis, 2006, 27, 2984; DOI: https://doi.org/10.1002/elps.200500730.
H. Nady, Egypt. J. Pet., 2017, 26, 905; DOI: https://doi.org/10.1016/j.ejpe.2016.02.004.
M. E. Zayed, R. A. Ammar, J. Saudi Chem. Soc., 2014, 18, 774; DOI: https://doi.org/10.1016/j.jscs.2011.08.006.
E. A. Hassan, N. Nawar, M. M. Mostafa, Appl. Organomet. Chem., 2019, e5096; DOI: https://doi.org/10.1002/aoc.5096.
L. Mosafa, M. Moghadam, M. Shahedi, Chinese J. Catal., 2013, 34, 1; DOI: https://doi.org/10.1016/S1872-2067(17)62892-4.
A. V. Pestov, P. A. Slepukhin, O. V. Koryakova, V. N. Charushin, Russ. J. Coord. Chem., 2014, 40, 216; DOI: https://doi.org/10.1134/s107032841404006x.
L. S. Molochnikov, A. V. Pestov, P. A. Slepukhin, Y. G. Yaltuk, Russ. J. Gen. Chem., 2009, 79, 1133; DOI: https://doi.org/10.1134/s1070363209060176.
A. V. Pestov, A. V. Virovets, N. V. Podberezskaya, Y. G. Yatluk, Russ. J. Coord. Chem., 2008, 34, 1; DOI: https://doi.org/10.1134/s1070328408010016.
M. I. Ul’yanova, S. A. Baskakova, T. V. Aksenova, P. A. Slepukhin, A. V. Pestov, Russ. J. Coord. Chem., 2015, 41, 240; DOI: https://doi.org/10.1134/s1070328415040090.
Russian National Standard GOST 10398-2016; https://docs.cntd.ru/document/1200141437 (in Russian).
V. P. Solov’ev, E. A. Vnuk, N. N. Strakhova, O. A. Raevskiy, Termodinamika kompleksoobrazovania soley shchelochnykh i shchelochnozemel’nykh metallov s tsiklicheskimi poliefirami [Thermodynamics of Complexation of Salts of Alkali and Alkaline Earth Metals with Cyclic Polyesters], VINITI, Moscow, 1991, 374 pp. (in Russian).
L. Alderighi, P. Gans, A. Ienco, D. Peters, A. Sabatini, A. Vacca, Coord. Chem. Rev., 1999, 184, 311; DOI: https://doi.org/10.1016/s0010-8545(98)00260-4.
I. Sovago, T. Kiss, A. Gergely, Pure Appl. Chem., 1993, 65, 1029; DOI: https://doi.org/10.1351/pac199365051029.
M. T. Beck, I. Nagypál, Chemistry of Complex Equilibria, Akadémiai Kiadó, Budapest, 1989, 402.
NIST Critically Selected Stability Constants of Metal Complexes, Version 8.0, 2004.
E. M. Abd-Alla, M. M. A. Mohamed, M. R. Mahmoud, J. Coord. Chem., 2003, 56, 691; DOI: https://doi.org/10.1080/0095897031000113995.
H. Irving, R. J. P. Williams, J. Chem. Soc., 1953, 3192; DOI:https://doi.org/10.1039/jr9530003192.
A. V. Kotov, J. Anal. Chem. USSR, 1988, 43, 741.
M. M. Khalil, A. M. Radalla, A. G. Mohamed, J. Chem. Eng. Data, 2009, 54, 3261; DOI: https://doi.org/10.1021/je9002459.
H. A. Azab, Z. M. Anwar, M. Sokar, J. Chem. Eng. Data, 2004, 49, 62; DOI: https://doi.org/10.1021/je0301702.
A. V. Pestov, E. V. Peresypkina, A. V. Virovets, N. V. Podberezskaya, Y. G. Yatluk, Y. A. Skorik, Acta Crystallogr. C, 2005, 61, m510; DOI: https://doi.org/10.1107/s0108270105033780.
N. E. Good, G. D. Winget, W. Winter, T. N. Connolly, S. Izawa, R. M. M. Singh, Biochemistry, 1966, 5, 467; DOI:https://doi.org/10.1021/bi00866a011.
Author information
Authors and Affiliations
Corresponding author
Additional information
Dedicated to Academician of the Russian Academy of Sciences O. M. Nefedov on the occasion of his 90th birthday.
Published in Russian in Izvestiya Akademii Nauk. Seriya Khimicheskaya, No. 1, pp. 152–157, January, 2022.
This work was fi nancially supported by the Government of the Russian Federation (Decree No. 211, Contract No. 02.A03.21.0006) and carried out within the framework of the state assignment to the I. Ya. Postovsky Institute of Organic Synthesis of the Ural Branch of the Russian Academy of Sciences (Topic No. AAAA-A19-119012290117-6) using the equipment of the Center for Collective Use “Spectroscopy and Analysis of Organic Compounds”.
No human or animal subjects were used in this research.
The authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Zharkov, G.P., Filimonova, O.V., Petrova, Y.S. et al. Complexing properties of N-[1,1-bis(hydroxymethyl)ethyl]-and N-[tris(hydroxymethyl)methyl]-β-alanine. Russ Chem Bull 71, 152–157 (2022). https://doi.org/10.1007/s11172-022-3389-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11172-022-3389-2