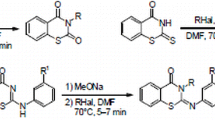
Abstract
Modification of phenothiazine and carbazole derivatives with trifluoromethyl-containing 1,3,5-oxadiazines and imidazolidinediones based on the copper(I)-catalyzed alkyne-azide 1,3-cycloaddition was carried out.
Similar content being viewed by others
References
A. Gluszynska, Eur. J. Med. Chem., 2015, 94, 405; DOI: https://doi.org/10.1016/j.ejmech.2015.02.059.
A. D. Mosnaim, V. V. Ranade, M. E. Wolf, J. Puente, M. A. Valenzuela, Am. J. Ther., 2006, 13, 261; DOI: https://doi.org/10.1097/01.mjt.0000212897.20458.63.
K. Pluta, B. Morak-Młodawska, M. Jelen, Eur. J. Med. Chem., 2011, 46, 3179; DOI: https://doi.org/10.1016/j.ejmech.2011.05.013.
R. G. Zenkov, L. V. Ektova, O. A. Vlasova, G. A. Belitskiy, M. G. Yakubovskaya, K. I. Kirsanov, Chem. Heterocycl. Compd. (Engl. Transl.), 2020, 56, 644; DOI: https://doi.org/10.1007/s10593-020-02714-4.
W. Murray, D. C. Rees, Nat. Chem., 2009, 1, 187; DOI: https://doi.org/10.1038/nchem.217.
M. G. Savelieff, G. Nam, J. Kang, H. J. Lee, M. Lee, M. H. Lim, Chem. Rev., 2019, 119, 1221; DOI: https://doi.org/10.1021/acs.chemrev.8b00138.
S. O. Bachurin, E. F. Shevtsova, G. F. Makhaeva, V. V. Grigoriev, N. P. Boltneva, N. V. Kovaleva, S. V. Lushchekina, P. N. Shevtsov, M. E. Neganova, O. M. Redkozubova, E. V. Bovina, A. V. Gabrelyan, V. P. Fisenko, V. B. Sokolov, A. Yu. Aksinenko, V. Echeverria, G. E. Barreto, G. Aliev, Sci. Rep., 2017, 45627; DOI: https://doi.org/10.1038/srep45627.
G. F. Makhaeva, S. V. Lushchekina, N. P. Boltneva, V. B. Sokolov, V. V. Grigoriev, O. G. Serebryakova, E. A. Vikhareva, A. Yu. Aksinenko, G. E. Barreto, G. Aliev, S. O. Bachurin, Sci. Rep., 2015, 13164; DOI: https://doi.org/10.1038/srep13164.
V. B. Sokolov, G. F. Makhaeva, A. Yu. Aksinenko, V. V. Grigoriev, E. F. Shevtsova, S. O. Bachurin, Russ. Chem. Bull., 2017, 66, 1821; DOI: https://doi.org/10.1007/s11172-017-1953-y.
S. O. Bachurin, E. P. Shevtsova, N. N. Lermontova, T. P. Serkova, R. R. Ramsay, Neurotoxicology, 1996, 17, 897.
G. F. Makhaeva, E. F. Shevtsova, N. V. Kovaleva, E. V. Rudakova, M. E. Neganova, L. G. Dubova, P. N. Shevtsov, A. Yu. Aksinenko, V. B. Sokolov, S. O. Bachurin, Russ. Chem. Bull., 2018, 67, 2121; DOI: https://doi.org/10.1007/s11172-018-2338-6.
V. B. Sokolov, A. Yu. Aksinenko, T. V. Goreva, T. A. Epishina, S. O. Bachurin, Russ. Chem. Bull., 2019, 68, 2241; DOI: https://doi.org/10.1007/s11172-019-2693-y.
A. Yu. Aksinenko, T. V. Goreva, T. A. Epishina, Russ. Chem. Bull., 2021, 70, 487; DOI: https://doi.org/10.1007/s11172-021-3113-7.
X. Cao, S. Ke, Rec. Adv. Med. Chem., 2015, 2, 107; DOI: https://doi.org/10.2174/97816810817171150201.
L. Konnert, F. Lamaty, J. Martinez, E. Colacino, Chem. Rev., 2017, 117, 13757; DOI: https://doi.org/10.1021/acs.chemrev.7b00067.
V. B. Sokolov, A. Yu. Aksinenko, Russ. Chem. Bull., 2013, 61, 2124; DOI: https://doi.org/10.1007/s11172-012-0297-x.
V. B. Sokolov, A. Yu. Aksinenko, A. V. Sokolov, A. V. Gabrelian, A. D. Efimova, V. V. Grigoriev, Russ. Chem. Bull., 2017, 66, 99; DOI: https://doi.org/10.1007/s11172-017-1706-y.
S. Bachurin, S. Tkachenko, I. Baskin, N. Lermontova, T. Mukhina, L. Petrova, A. Ustinov, A. Proshin, V. Grigoriev, N. Lukoyanov, V. Palyulin, N. Zefirov, Ann. N.-Y. Acad. Sci., 2001, 939, 219; DOI: https://doi.org/10.1111/j.1749-6632.2001.tb03629.x.
S. O. Bachurin, S. I. Gavrilova, A. Samsonova, G. E. Barreto, G. Aliev, Pharmacol. Res., 2018, 129, 216; DOI: https://doi.org/10.1016/j.phrs.2017.11.021.
A. Yu. Aksinenko, T. V. Goreva, T. A. Epishina, A. N. Pushin, V. B. Sokolov, Russ. Chem. Bull., 2006, 55, 1052; DOI: https://doi.org/10.1007/s11172-006-0375-z.
V. B. Sokolov, A. Yu. Aksinenko, T. A. Epishina, T. V. Goreva, I. V. Martynov, Russ. Chem. Bull., 2005, 54, 2851; DOI: https://doi.org/10.1007/s11172-006-0200-8.
C. Sae-Lim, D. J. Sandman, B. M. Foxman, M. Sukwat-tanasinitt, J. Macromol. Sci. (A), 2006, 43, 1929; DOI: https://doi.org/10.1080/10601320600996114.
K. Kolli, B. Prasad, P. V. Babu, M. A. Ashfaq, N. Z. Ehtesham, R. R. Raju, M. Pal, Org. Biomol. Chem., 2014, 12, 6080; DOI: https://doi.org/10.1039/c4ob00686k.
V. B. Sokolov, A. Yu. Aksinenko, T. A. Epishina, T. V. Goreva, Russ. Chem. Bull., 2018, 67, 1679; DOI: https://doi.org/10.1007/s11172-018-2276-3.
Funding
This work was financially supported by the Russian Science Foundation (Project No. 19-13-00378). The research was carried out using equipment of the Center for Collective use of the Institute of Physiologically Active Compounds of the Russian Academy of Sciences.
Author information
Authors and Affiliations
Corresponding author
Additional information
Dedicated to Academician of the Russian Academy of Sciences O. M. Nefedov on the occasion of his 90th birthday.
Published in Russian in Izvestiya Akademii Nauk. Seriya Khimicheskaya, No. 11, pp. 2180–2184, November, 2021.
This paper does not contain descriptions of studies on animals or humans.
The authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Aksinenko, A.Y., Sokolov, V.B., Gabrel’yan, A.V. et al. Modification of phenothiazine and carbazole derivatives with trifluoromethyl-containing 1,3,5-oxadiazines and imidazolidinediones. Russ Chem Bull 70, 2180–2184 (2021). https://doi.org/10.1007/s11172-021-3329-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11172-021-3329-6