Abstract
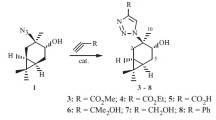
An efficient synthesis of 7-substituted 3-azabicyclo[3.3.1]non-6-enes from available 3-borabicyclo[3.3.1]non-6-enes, the products of allylboron-acetylene condensation, was developed. The terminal alkynes with nitrogen-containing substituents were involved for the first time into the reaction with triallylborane.
Similar content being viewed by others
Change history
31 January 2024
An Erratum to this paper has been published: https://doi.org/10.1007/s11172-023-4117-2
References
M. A. Turabekova, B. F. Rasulev, F. N. Dzhakhangirov, D. Leszczynska, J. Leszczynski, Eur. J. Med. Chem., 2010, 45, 3885; DOI: https://doi.org/10.1016/j.ejmech.2010.05.042.
S. Song, Q. Tang, H. Huo, H. Li, X. Xing, J. Luo, J. Analyt. Toxicology, 2015, 39, 58; DOI: https://doi.org/10.1093/jat/bku113.
Z.-G. Liu, H. Cheng, M.-J. Ge, L. Xu, F.-P. Wang, Tetrahedron, 2013, 69, 5431; DOI: https://doi.org/10.1016/j.tet.2013.04.102.
J. J. Sahn, P. Bharathi, D. L. Comins, Tetrahedron Lett., 2012, 53, 1347; DOI: https://doi.org/10.1016/j.tetlet.2011.12.127.
J. W. Coe, P. R. Brooks, M. G. Vetelino, M. C. Wirts, E. P. Arnold, J. Huang, S. B. Sands, T. I. Davis, L. A. Label, C. B. Fox, A. Shrikhande, J. H. Heym, E. Schaeffer, H. Rollema, Y. Lu, R. S. Mansbach, L. K. Chambers, C. C. Rovetti, D. W. Schulz, F. D. Tingley, B. T. O’Neill, J. Med. Chem., 2005, 48, 3474; DOI: https://doi.org/10.1021/jm050069n.
H. Rolemma, J. W. Coe, L. K. Chambers, R. S. Hurst, S. M. Stahl, K. E. Williams, Trends Pharmacol. Sci., 2007, 28, 316; DOI: https://doi.org/10.1016/j.tips.2007.05.003.
R. Jeyaraman, S. Avila, Chem. Rev., 1981, 81, 149; DOI: https://doi.org/10.1021/cr00042a002.
B. M. Mikhailov, Yu. N. Bubnov, Bull. Acad. Sci. USSR, Div. Chem. Sci., 1965, 14, 1285; DOI: https://doi.org/10.1007/BF00847925.
Yu. N. Bubnov, T. V. Potapova, M. E. Gursky, J. Organomet. Chem., 1991, 412, 311; DOI: https://doi.org/10.1016/0022-328X(91)86075-2.
F. N. Shirota, E. G. DeMaster, J. A. Elberling, H. T. Nagasawa, J. Med. Chem., 1980, 23, 669; DOI: https://doi.org/10.1021/jm00180a018.
G. A. Russell, P. Ngoviwatchai, Y. W. Wu, J. Am. Chem. Soc., 1989, 111, 4921; DOI: https://doi.org/10.1021/ja00195a054.
Yu. N. Bubnov, S. I. Frolov, V. G. Kiselev, B. M. Mikhailov, J. Gen. Chem. USSR, 1970, 40, 1307.
B. M. Mikhailov, T. K. Baryshnikova, Bull. Acad. Sci. USSR, Div. Chem. Sci., 1979, 28, 2358; DOI: https://doi.org/10.1007/BF00951715.
B. M. Mikhailov, T. K. Baryshnikova, V. G. Kiselev, A. S. Shashkov, Bull. Acad. Sci. USSR, Div. Chem. Sci., 1979, 28, 2361; DOI: https://doi.org/10.1007/BF00951716.
B. M. Mikhailov, Yu. N. Bubnov, Bororganicheskie soedineniyav organicheskom sinteze [Organoboron Compounds in Organic Synthesis], Nauka, Moscow, 1977, 516 pp. (in Russian).
N. Yu. Kuznetsov, Z. A. Starikova, B. B. Averkiev, Yu. N. Bubnov, Russ. Chem. Bull., 2005, 54, 678; DOI: https://doi.org/10.1007/s11172-005-0305-5.
A. L. Semenova, Ph. D. Thesis (Chem.), N. D. Zelinsky Institute of Organic Chemistry of the Russian Academy of Sciences, Moscow, 2006, 156 pp. (in Russian).
K. J. Goodall, M. A. Brimble, D. Barker, Tetrahedron, 2012, 68, 5759; DOI: https://doi.org/10.1016/j.tet.2012.05.037.
M. E. Gurskii, G. D. Kolomnikova, S. V. Baranin, Yu. N. Bubnov, Mendeleev Commun., 2018, 28, 366; DOI: https://doi.org/10.1016/j.mencom.2018.07.008.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Russian in Izvestiya Akademii Nauk. Seriya Khimicheskaya, No. 10, pp. 1987–1993, October, 2021.
The work was carried out within the framework of the program for the development of scientific schools of the N. D. Zelinsky Institute of Organic Chemistry of the Russian Academy of Sciences.
This paper does not contain descriptions of studies on animals or humans.
The authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Iurenkov, M.V., Potapova, T.V., Baranin, S.V. et al. Design of 7-substituted 3-azabicyclo[3.3.1]non-6-enes based on allylboron-acetylene condensation. Russ Chem Bull 70, 1987–1993 (2021). https://doi.org/10.1007/s11172-021-3306-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11172-021-3306-0