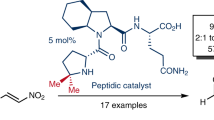
Abstract
A comprehensive study of the activity of the amide-type organocatalysts based on (R)- and (S)-phenylglycine and 1,2-di(2-pyridyl)-1,2-diaminoethane in the asymmetric Michael reaction between various nitroalkenes and ketones was carried out. The products of the studied reactions were formed in up to 99% yield, with syn diastereoselectivity (dr) >20: 1 and enantiomeric excess of up to 93% ee for syn-isomer. The organocatalysts can be regenerated and reused in at least seven reaction cycles.
Similar content being viewed by others
References
G. Zhan, W. Du, Y. C. Chen, Chem. Soc. Rev., 2017, 46, 1675–1692; DOI: https://doi.org/10.1039/C6CS00247A.
J. Alemán, S. Cabrera, Chem. Soc. Rev., 2013, 42, 774–793; DOI: https://doi.org/10.1039/C2CS35380F.
T. James, M. Van Gemmeren, B. List, Chem. Rev., 2015, 115, 9388–9409; DOI: https://doi.org/10.1021/acs.chemrev.5b00128.
J. Y. Du, Y. H. Ma, F. X. Meng, R. R. Zhang, R. N. Wang, H. L. Shi, Q. Wang, Y. X. Fan, H. L. Huang, J. C. Cui, C. L. Ma, Org. Lett., 2019, 21, 465–468; DOI: https://doi.org/10.1021/acs.orglett.8b03709.
D. S. Ji, Y. C. Luo, X. Q. Hu, P. F. Xu, Org. Lett., 2020, 22, 1028–1033; DOI: https://doi.org/10.1021/acs.orglett.9b04571.
K. N. Gavrilov, I. V. Chuchelkin, V. K. Gavrilov, S. V. Zheglov, I. D. Firsin, V. M. Trunina, A. V. Maximychev, A. M. Perepukhov, Russ. Chem. Bull., 2021, 70, 336.
K. N. Gavrilov, S. V. Zheglov, V. K. Gavrilov, I. D. Firsin, M. G. Maksimova, Russ. Chem. Bull., 2019, 68, 1376; DOI: https://doi.org/10.1007/s11172-019-2564-6.
U. Scheffler, R. Mahrwald, Chem. — Eur. J., 2013, 19, 14346–14396; DOI: https://doi.org/10.1002/chem.201301996.
V. Kozma, F. Fülöp, G. Szőllősi, Adv. Synth. Catal., 2020, 362, 2444–2458; DOI: https://doi.org/10.1002/adsc.202000335.
T. L. da Silva, R. S. Rambo, C. G. Jacoby, P. H. Schneider, Tetrahedron, 2020, 76, 130874; DOI: https://doi.org/10.1016/j.tet.2019.130874.
A. Y. Sukhorukov, A. A. Sukhanova, S. G. Zlotin, Tetrahedron, 2016, 72, 6191–6281; DOI: https://doi.org/10.1016/j.tet.2016.07.067.
K. O. Eyong, H. L. Ketsemen, Z. Zhao, L. Du, A. Ingels, V. Mathieu, A. Kornienko, K. G. Hull, G. N. Folefoc, S. Baskaran, D. Romo, Med. Chem. Res., 2020, 29, 1058–1066; DOI: https://doi.org/10.1007/s00044-020-02545-0.
Y. Wang, D. M. Du, Org. Chem. Front., 2020, 7, 3266–3283; DOI: https://doi.org/10.1039/D0QO00631A.
D. Roca-Lopez, D. Sadaba, I. Delso, R. P. Herrera, T. Tejero, P. Merino, Tetrahedron: Asymmetry, 2010, 21, 2561–2601; DOI: https://doi.org/10.1016/j.tetasy.2010.11.001.
K. A. Bykova, A. A. Kostenko, A. S. Kucherenko, S. G. Zlotin, Russ. Chem. Bull., 2019, 68, 1402; DOI: https://doi.org/10.1007/s11172-019-2568-2.
A. S. Kucherenko, V. G. Lisnyak, A. A. Kostenko, S. V. Kochetkov, S. G. Zlotin, Org. Biomol. Chem., 2016, 14, 9751–9759; DOI: https://doi.org/10.1039/C6OB01606E.
K. M. Koeller, C. H. Wong, Nature, 2001, 409, 232–240; DOI:10.1038/35051706.
G. Carrea, S. Riva, Angew. Chem., Int. Ed., 2000, 39, 2226–2254; DOI: https://doi.org/10.1002/1521-3773(20000703)39:13%3C2226::AID-ANIE2226%3E3.0.CO;2-L.
P. J. Um, D. G. Drueckhammer, J. Am. Chem. Soc., 1998, 120, 5605–5610; DOI: https://doi.org/10.1021/ja980445b.
K. Sakthivel, W. Notz, T. Bui, C. F. Barbas, J. Am. Chem. Soc., 2001, 123, 5260–5267; DOI: https://doi.org/10.1021/ja010037z.
L. W. Xu, J. Luo, Y. Lu, Chem. Commun., 2009, 1807–1821; DOI: https://doi.org/10.1039/B821070E.
O. V. Serdyuk, C. M. Heckel, S. B. Tsogoeva, Org. Biomol. Chem., 2013, 11, 7051–7071; DOI: https://doi.org/10.1039/C3OB41403E.
V. G. Lisnyak, A. S. Kucherenko, E. F. Valeev, S. G. Zlotin, J. Org. Chem., 2015, 80, 9570–9577; DOI: https://doi.org/10.1021/acs.joc.5b01555.
S. P. Chavan, S. Garai, K. P. Pawar, Tetrahedron Lett., 2013, 54, 2137–2139; DOI: https://doi.org/10.1016/j.tetlet.2013.02.029.
C. Xu, J. Du, L. Ma, G. Li, M. Tao, W. Zhang, Tetrahedron, 2013, 69, 4749–4757; DOI: https://doi.org/10.1016/j.tet.2013.02.084.
H. Huang, E. N. Jacobsen, J. Am. Chem. Soc., 2006, 128, 7170–7171; DOI: https://doi.org/10.1021/ja0620890.
Author information
Authors and Affiliations
Corresponding author
Additional information
Dedicated to Academician of the Russian Academy of Sciences V. N. Charushin on the occasion of his 70th birthday.
Published in Russian in Izvestiya Akademii Nauk. Seriya Khimicheskaya, No. 5, pp. 885–889, May, 2021.
Rights and permissions
About this article
Cite this article
Kostenko, A.A., Kuznetsova, O.Y., Kucherenko, A.S. et al. Novel C2-symmetric phenylglycine derivatives as organocatalysts of the Michael reaction between nitroalkenes and ketones. Russ Chem Bull 70, 885–889 (2021). https://doi.org/10.1007/s11172-021-3163-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11172-021-3163-x