Abstract
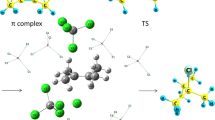
On the basis of studying the reactivities of the initial and intermediate compounds in the acyl chloride—water system, methods for controlling the reaction by means of arising hydrogen bonds were found. Particular reaction steps affecting the energy profile of the reaction were elucidated. The structural and energy characteristics of the stable conformers of compounds and the energies of the ground and transition states were determined using the B3LYP/aug-cc-pVDZ method. The autocatalysis of the reaction by its products, acetic acid and hydrogen chloride, was discussed.
Similar content being viewed by others
References
Non-covalent Interactions in the Synthesis and Design of New Compounds, Eds A. M. Maharramov, K. T. Mahmudov, M. N. Kopylovich, A. J. L. Pombeiro, John Wiley & Sons, New Jersey, 2016, 480 pp.; DOI: https://doi.org/10.1002/9781119113874.
Noncovalent Interactions in Catalysis, Eds K. T. Mahmudov, M. N. Kopylovich, M. F. C. G. da Silva, A. J. L. Pombeiro, Royal Society of Chemistry, Cambridge, 2019, 653 pp.; DOI: https://doi.org/10.1039/9781788016490.
Tablitsy konstant skorostei i ravnovesiya geteroliticheskikh organicheskikh reaktsii [Tables of Rate and Equilibrium Constants of Heterolytic Organic Reactions], Ed. V. A. Palm, 3(II), VINITI, Moscow, 1977, 591 pp. (in Russian).
J. Koskikallio, Suomen Kemistilehti, 1962, 35, 62.
R. J. E. Talbot, in Comprehensive Chemical Kinetics, Elsevier, New York, 1972, 10, 209; DOI: https://doi.org/10.1016/S0069-8040(08)70345-5.
M. L. Bender, M. C. Chen, J. Am. Chem. Soc., 1963, 85, 30; DOI: https://doi.org/10.1021/ja00884a006.
V. A. Terentev, V. V. Varfolomeeva, Russ. J. Gen. Chem., 1996, 66, 2010.
V. A. Terentev, V. V. Varfolomeeva, Russ. J. Gen. Chem., 1996, 66, 293.
V. A. Terentev, V. V. Varfolomeeva, Russ. J. Gen. Chem., 2000, 70, 430.
A. N. Nesmeyanov, N. A. Nesmeyanov, Nachala organicheskoi khimii [Fundamentals of Oganic Chemistry], Khimiya, Moscow, 1969, 663 pp. (in Russian).
T. W. Bentley, C. S. Shim, J. Chem. Soc., Perkin Trans. 2, 1993, 9, 1659; DOI: https://doi.org/10.1039/P29930001659.
K. Burke, J. Chem. Phys., 2012, 136, 150901; DOI: https://doi.org/10.1063/1.4704546.
V. V. Varfolomeeva, A. V. Terentev, Phys. Chem. Chem. Phys., 2015, 17, 24282; DOI: https://doi.org/10.1039/c5cp04295.
A. Srivastava, R. Mishra, S. Kumar, K. Dev, P. Tandon, R. Maurya, J. Mol. Struct., 2015, 1084, 55; DOI: https://doi.org/10.1016/j.molstruc.2014.11.070.
E. Nazarparvar, M. Zahedi, E. Klein, J. Org. Chem., 2012, 77, 10093; DOI: https://doi.org/10.1021/jo301612a.
B. G. Oliveira, R. C. M. U. Araújo, A. B. Carvalho, M. N. Ramos, J. Mol. Model., 2009, 15, 421; DOI: https://doi.org/10.1007/s00894-008-0422-9.
N. Mardirossian, M. Head-Gordon, Mol. Phys., 2017, 115, 2315; DOI: https://doi.org/10.1080/00268976.2017.1333644.
Y. Zhao, N. Gonzalez-Garcia, D. G. Truhlar, J. Phys. Chem. A, 2005, 109, 2012; DOI: https://doi.org/10.1021/jp045141s.
J. Zheng, Y. Zhao, D. G. Truhlar, J. Chem. Theory Comput., 2009, 5, 808; DOI: https://doi.org/10.1021/ct800568m.
CCCBDB: Computational Chemistry Comparison and Benchmark Data Base. NIST Standard Reference Database Number 101 Release 18, October 2016, Ed. R. D. Johnson III; http://cccbdb.nist.gov.
A. A. Granovsky, Fire fly version 8;http://classic.chem.msu.su/gran/firefly/index.html.
M. W. Schmidt, K. K. Baldridge, J. A. Boatz, S. T. Elbert, M. S. Gordon, J. H. Jensen, S. Koseki, N. Matsunaga, K. A. Nguyen, S. Su, T. L. Windus, M. Dupuis, J. A. Montgomery, J. Comput. Chem., 1993, 14, 1347; DOI: https://doi.org/10.1002/jcc.540141112.
E. J. Hartwell, R. E. Richards, H. W. Thompson, J. Chem. Soc., 1948, 1436; DOI: https://doi.org/10.1039/JR9480001436.
D. Cook, J. Am. Chem. Soc., 1958, 80, 49; DOI: https://doi.org/10.1021/ja01534a014.
V. A. Terentev, V. V. Varfolomeeva, Russ. J. Gen. Chem., 1994, 64, 98.
NIST Chemistry Web Book, NIST Standard Reference Database Number 69, 2018; DOI: https://doi.org/10.18434/T4D303.
H. M. Randall, R. G. Fowler, N. Fuson, J. R. Dangl, Infrared Determination of Organic Structures, Van Nostrand Company, 1949, 239 pp.
M. B. Smith, March’s Advanced Organic Chemistry. Reaction, Mechanisms, and Structure, Wiley, 2013, 2080 pp.
E. K. Euranto, L. T. Kanerva, Acta Chem. Scand. Ser. B., 1998, 42, 717; DOI: https://doi.org/10.3891/acta.chem.scand.42b-0717.
R. T. Morrison, R. S. Boyd, Organic Chemistry, Allyn and Bacon, 1973, 1204 pp.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Russian in Izvestiya Akademii Nauk. Seriya Khimicheskaya, No. 4, pp. 693–698, April, 2021.
This paper does not contain descriptions of studies on animals or humans.
The authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Varfolomeeva, V.V., Terentev, A.V. Effect of hydrogen bond on the mechanism of acyl chloride hydrolysis. Russ Chem Bull 70, 693–698 (2021). https://doi.org/10.1007/s11172-021-3138-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11172-021-3138-y