Abstract
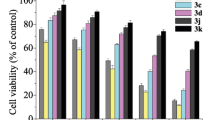
Synthesis of new bis(seleno-imidazolone) derivatives bearing the alkyl and aryl moieties at the N(1) atom of the five-membered ring was developed. Cytotoxicity of the synthesized compounds against A549, VA-13, MCF-7, and HEK293T cell lines was evaluated.
Similar content being viewed by others
References
G. Mugesh, H. Singh, Chem. Soc. Rev., 2000, 29, 347; DOI: https://doi.org/10.1039/A908114C.
C. Narajji, M. D. Karvekar, A. K. Das, Indian J. Pharm. Sci., 2007, 69, 344; DOI: https://doi.org/10.4103/0250-474X.34541.
D. Plano, Y. Baquedano, E. Ibánez, I. Jiménez, J. A. Palop, J. E. Spallholz, C. Sanmartín, Molecules, 2010, 15, 7292; DOI: https://doi.org/10.3390/molecules15107292.
C. F. Bortolatto, P. M. Chagas, E. A. Wilhelm, G. Zeni, C. W. Nogueira, J. Enzyme Inhib., 2013, 28, 677; DOI: https://doi.org/10.3109/14756366.2012.670805.
S. T. Stefanello, A. S. Prestes, T. Ogunmoyole, S. M. Salman, R. S. Schwab, C. R. Brender, L. Dornelles, J. B. T. Rocha, F. A. A. Soares, Toxicol. in Vitro, 2013, 27, 1433; DOI: https://doi.org/10.1016/j.tiv.2013.03.001.
A. C. Sauer, J. G. Leal, S. T. Stefanello, M. T. B. Leite, M. B. Souza, F. A. A. Soares, O. E. D. Rodrigues, L. Dornelles, Tetrahedron Lett., 2017, 58, 87; DOI: https://doi.org/10.1016/j.tetlet.2016.11.106.
K. El-Bayoumy, Cancer Res., 1985, 45, 3631; https://cancerres.aacrjournals.org/content/45/8/3631.short.
P. Sahu, G. Kim, J. Yu, J. Ahn, J. Song, Y. Choi, X. Jin, J. Kim, S. Lee, S. Park, L. Jeong, Org. Lett., 2014, 16, 5796; DOI: https://doi.org/10.1021/ol502899b.
M. Radhakrishna, C. Sharadamma, M. Vagdevi, M. Abhilekha, S. Rubeena, K. Nischal, Int. J. Chem., 2010, 2, 149; DOI: https://doi.org/10.5539/ijc.v2n2p149.
G. Roy, G. Mugesh, J. Am. Chem. Soc., 2005, 127, 15207; DOI: https://doi.org/10.1021/ja054497u.
C. Nogueira, G. Zeni, J. Rocha, Chem. Rev., 2004, 104, 6255; DOI: https://doi.org/10.1021/cr0406559.
O. Epp, R. Ladenstein, A. Wendel, Eur. J. Biochem., 1983, 133, 51; DOI: https://doi.org/10.1111/j.1432-1033.1983.tb07429.x.
A. A. Lysova, R. D. Marchenko, D. G. Samsonenko, A. S. Potapov, V. P. Fedina, Russ. Chem. Bull., 2020, 69, 1122; DOI: https://doi.org/10.1007/s11172-020-2877-5.
Y. Sun, M.-W. Ding, Chin. J. Synth. Chem., 2005, 13, 546, 590.
A. A. El-Barbary, A. A. Saffan, M. A. Sakran, A. I. Khodair, Delta J. Sci., 1990, 14, 601.
E. K. Beloglazkina, A. G. Majouga, I. V. Yudin, N. V. Zyk, A. A. Moiseeva, K. P. Butin, Russ. Chem. Bull., 2005, 54, 2163; DOI: https://doi.org/10.1007/s11172-007-0057-5.
E. K. Beloglazkina, A. G. Majouga, R. B. Romashkina, A. A. Moiseeva, N. V. Zyk, Polyhedron, 2007, 26, 797; DOI: https://doi.org/10.1016/j.poly.2006.09.012.
A. G. Majouga, E. K. Beloglazkina, O. V. Shilova, A. A. Moiseeva, N. V. Zyk, Russ. Chem. Bull., 2009, 58, 1392; DOI: https://doi.org/10.1007/s11172-009-0185-1.
E. K. Beloglazkina, A. G. Majouga, A. V. Mironov, A. V. Yudina, O. Yu. Kuznetsova, N. V. Zyk, Polyhedron, 2014, 76, 45; DOI: https://doi.org/10.1016/j.poly.2014.03.045.
A. Majouga, M. Zvereva, M. Rubtsova, D. Skvortsov, A. Mironov, D. Azhibek, O. Krasnovskaya, V. Gerasimov, A. Udina, N. Vorozhtsov, E. Beloglazkina, L. Agron, L. Mikhina, A. Tretyakova, N. Zyk, N. Zefirov, A. Kabanov, O. Dontsova, J. Med. Chem., 2014, 57, 6252; DOI: https://doi.org/10.1021/jm500154f.
E. K. Beloglazkina, O. O. Krasnovskaya, D. A. Guk, V. A. Tafeenko, A. A. Moiseeva, N. V. Zyk, A. G. Majouga, Polyhedron, 2018, 148, 129; DOI: https://doi.org/10.1016/j.poly.2018.04.005.
O. O. Krasnovskaya, D. A. Guk, A. E. Naumov, V. N. Nikitina, A. S. Semkina, K. Yu. Vlasova, V. Pokrovsky, O. O. Ryabaya, S. S. Karshieva, D. A. Skvortsov, I. V. Zhirkina, R. R. Shafikov, P. V. Gorelkin, A. N. Vaneev, A. S. Erofeev, D. M. Mazur, V. A. Tafeenko, V. I. Pergushov, M. Ya. Melnikov, M. A. Soldatov, V. V. Shapovalov, A. V. Soldatov, R. A. Akasov, V. M. Gerasimov, D. A. Sakharov, A. A. Moiseeva, N. V. Zyk, E. K. Beloglazkina, A. G. Majouga, J. Med. Chem., 2020, 63, 13031; DOI: https://doi.org/10.1021/acs.jmedchem.0c01196.
Y. Sun, L. Gao, M. Ding, Heterocycl. Commun., 2005, 11, 69; DOI: https://doi.org/10.1515/HC.2005.11.1.69.
M. Yu. Steklov, A. N. Chernysheva, R. L. Antipin, A. G. Majouga, E. K. Beloglazkina, A. A. Moiseeva, E. D. Strel’tsova, N. V. Zyk, Russ. Chem. Bull., 2012, 61, 1182; DOI: https://doi.org/10.1007/slll72-012-0161-z].
Y. A. Ivanenkov, M. S. Veselov, I. G. Rezekin, D. A. Skvortsov, Y. B. Sandulenk, M. V. Polyakova, D. S. Bezrukov, S. V. Vasilevsky, M. E. Kukushkin, A. A. Moiseeva, A. V. Finko, V. E. Koteliansky, N. L. Klyachko, L. A. Filatova, E. K. Beloglazkina, N. V. Zyk, A. G. Majouga, Bioorg. Med. Chem., 2016, 24, 802; DOI: https://doi.org/10.1016/j.bmc.2015.12.050.
V. K. Novotortsev, M. E. Kukushkin, V. A. Tafeenko, N. V. Zyk, E. K. Beloglazkina, Mendeleev Commun., 2020, 30, 320; DOI: https://doi.org/10.1016/j.mencom.2020.05.020.
O. Vyhivskyi, E. A. Dlin, A. V. Finko, S. P. Stepanova, Y. A. Ivanenkov, D. A. Skvortsov, A. V. Mironov, N. V. Zyk, A. G. Majouga, E. K. Beloglazkina, ACS Comb. Sci., 2019, 21, 456; DOI: https://doi.org/10.1021/acscombsci.9b00021.
O. Vyhivskyi, D. N. Laikov, A. V. Finko, D. A. Skvortsov, I. V. Zhirkina, V. A. Tafeenko, N. V. Zyk, A. G. Majouga, E. K. Beloglazkina, J. Org. Chem., 2020, 85, 3160; DOI: https://doi.org/10.1021/acs.joc.9b03045.
L. A. Wessjohann, A. Schneider, M. Abbas, W. Brandt, Biol. Chem., 2007, 388, 997; DOI: https://doi.org/10.1515/BC.2007.138.
N. Sonoda, A. Ogawa, F. Recupero, in Encyclopedia of Reagents for Organic Synthesis, 2005, 1–7; DOI: https://doi.org/10.1002/047084289X.rb018.pub2.
T. Mosmann, J. Immunol. Methods, 1983, 65, 55; DOI: https://doi.org/10.1016/0022-1759(83)90303-4.
E. S. Antonarakis, Transl. Androl. Urol., 2013, 2, 119; DOI: https://doi.org/10.3978/j.issn.2223-4683.2012.09.04
S. Payton, Nat. Rev. Urol., 2014, 11, 243; DOI: https://doi.org/10.1038/nrurol.2014.98.
D. M. Moran, C. G. Maki, Mol. Cancer. Ther., 2010, 9, 895; DOI: https://doi.org/10.1158/1535-7163.MCT-09-1220.
H. Shen, C. G Maki, J. Biol. Chem., 2010, 285, 23105; DOI: https://doi.org/10.1074/jbc.M110.124990.
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was performed within the framework of the State assignment of Lomonosov Moscow State University using the equipment of Center of the collective usage of MSU.
This paper does not contain descriptions of studies on animals or humans.
The authors declare no competing interests.
Published in Russian in Izvestiya Akademii Nauk. Seriya Khimicheskaya, No. 3, pp. 457–462, March, 2021.
Rights and permissions
About this article
Cite this article
Finko, A.V., Sokolov, A.I., Vasilyeva, L.A. et al. Synthesis of 4,4′-substituted 2,2′-[ethane-1,2-diylbis(selanediyl)]bis(1H-imidazol-5(4H)-ones). Russ Chem Bull 70, 457–462 (2021). https://doi.org/10.1007/s11172-021-3108-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11172-021-3108-4