Abstract
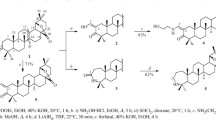
The synthesis of 2- and 3-hydroxy derivatives of isocembrol was accomplished by the epoxidation of isocembrol with tert-butyl hydroperoxide in the presence of a catalytic amount of VO(acac)2 followed by the epoxide ring opening in the presence of LiAlH4. The 13-hydroxy derivative of isocembrol was synthesized by the allylic oxidation in the SeO2-ButOOH system. Using the PASS software, the 13-hydroxy derivative was predicted to be the most effective cytotoxic agent, which was esterified to form N-methylurocanate. Isocembrol exhibited the highest in vitro antitumor activity against the LOX IMVI (melanoma) and UO-31 (renal cancer) cells, causing up to 25.77 and 75.65% cell death, respectively. N-Methylurocanate led to the death of up to 51.04% UO-31 cells (renal cancer).
Similar content being viewed by others
References
J. E. Burks, D. V. Helm, C. Y. Chonge, L. C. Ciereszko, Acta Crystallogr., Sect. B, 1972, 33, 704.
B. Tursch, J. C. Braekman, D. Daloze, M. Herin, R. Karlsson, Tetrahedron, 1975, 31, 129.
A. J. Weinheimer, J. A. Matson, M. B. Hossain, D. V. Helm, Tetrahedron Lett., 1977, 34, 2923.
V. A. Raldugin, V. E. Kozlov, V. M. Chekurov, N. I. Yaroshenko, V. A. Petegova, Chem. Nat. Compd., 1981, 17, 530.
A. J. Weinheimer, J. A. Matson, M. Poling, D. V. Helm, Acta Crystallogr., 1982, 38, 580.
M. Aoki, Y. Tooyama, T. Uyehara, T. Kato, Tetrahedron Lett., 1983, 24, 2267.
R. S. Jacobs, P. Culver, R. Lagdon, T. O’Brien, S. White, Tetrahedron, 1985, 41, 981.
J. C. Coll, B. F. Bowden, D. M. Tapiolas, R. H. Willis, P. Djura, M. Streamer, L. Trott, Tetrahedron, 1985, 41, 1085.
Y. Uchio, S. Eguchi, J. Kuramato, M. Nakayama, T. Hase, Tetrahedron Lett., 1985, 26, 4487.
M. A. Tius, Chem. Rew., 1988, 88, 719.
A. J. Weinheimer, J. A. Matson, M. Poling, Tetrahedron Lett., 1989, 30, 3491.
J. E. McMurry, J. G. Rico, Y. N. Shin, Tetrahedron Lett., 1989, 30, 1173.
U. Anthonu, K. Bock, C. Christophersen, J. O. Duus, E. V. Kjer, P. H. Nielsen, Tetrahedron Lett., 1991, 32, 2825.
N. J. G. Cox, S. D. Millas, G. Pattenden, J. Chem. Soc., Perkin Trans. 1, 1992, 1313.
A. D. Rodriguez, Y. Li, H. Dhasmana, J. Nat. Prod., 1993, 56, 564.
A. D. Rodriguez, Y. Li, H. Dhasmana, C. L. Barnes, J. Nat. Prod., 1993, 56, 1101.
A. D. Rodriguez, I. C. Pina, J. Org. Chem., 1995, 60, 8096.
C.-Y. Duh, S.-W. Wang, H.-K. Tseng, J.-H. Sheu, Tetrahedron Lett., 1998, 39, 7121.
W. D. Li, Y. Li, Tetrahedron Lett., 1999, 40, 965.
A. Fontana, M. L. Ciavatta, P. Amodeo, G. Cimino, Tetrahedron, 1999, 55, 1143.
D. J. Faulkner, Nat. Prod. Rep., 2000, 17, 7.
Z. Liu, L. Peng, W. Li, Y. Li, Synlett, 2003, 13, 1977.
R. A. Epifanio, B. A. Gama, R. C. Periera, Biochem. System Ecology, 2006, 34, 446.
A. J. Welford, J. J. Caldwell, M. Liu, M. Richards, N. Brown, C. Lomas, G. J. Tizzard, M. B. Pitak, S. J. Coles, S. A. Eccles, F. I. Raynaud, I. Collins, Chem. Eur. J., 2016, 22, 5657.
P. Bernardelli, L. A. Paquette, Heterocycles, 1998, 49, 531.
P.-J. Sung, M.-Ch. Chen, Heterocycles, 2002, 57, 1705.
Sh. J. Stachel, K. Biswas, S. J. Danishefsky, Curr. Pharm. Des., 2001, 7, 1277.
T. Lindel, P. R. Jensen, W. Fenical, B. H. Long, A. M. Casazza, J. Carboni, C. R. Fairchild, J. Am. Chem. Soc., 1997, 119, 8744.
T. Lindel, Angew. Chem., 1998, 37, 774.
K. C. Nicolaou, J.-Y. Xu, S. Kim, T. Ohshima, S. Hosokawa, J. Pfefferkorn, J. Am. Chem. Soc., 1997, 119, 11353.
K. C. Nicolaou, F. Roschangar, D. Vourloumis, Angew. Chem., Int. Ed. Engl., 1998, 37, 2014.
K. C. Nicolaou, N. Winssinger, D. Vourloumis, T. Ohshima, S. Kim, J. Pfefferkorn, J. Xu, T. Li, J. Am. Chem. Soc., 1998, 120, 10814.
K. C. Nicolaou, D. Vourloumis, T. Li, J. Pastor, N. Winssinger, Yu. He, S. Ninkovic, F. Sarabia, H. Vallberg, F. Roschangar, N. P. King, M. Ray V. Finlay, P. Giannakakou, P. Verdier-Pinard, E. Hamel, Angew. Chem., Int. Ed., 1997, 36, 2097.
K. C. Nicolaou, T. Ohshima, S. Hosokawa, F. V. Delft, D. Vourloumis, J. Xu, J. Pfefferkorn, S. Kim, J. Am. Chem. Soc., 1998, 120, 8674.
K. C. Nicolaou, J.-Y. Xu, S. Kim, J. Pfefferkorn, T. Ohshima, D. Vourloumis, S. Hosokawa, J. Am. Chem. Soc., 1998, 120, 8661.
X.-T. Chen, S. K. Bhattacharya, B. Zhou, C. E. Gutteridge, R. R. Pettus Thomas, S. J. Danishefsky, J. Am. Chem. Soc., 1999, 121, 6563.
K. C. Nicolaou, F. V. Delft, T. Ohshima, D. Vourloumis, J. Xu, S. Hosokawa, J. Pfefferkorn, S. Kim, T. Li, Angew. Chem., Int. Ed., 1997, 36, 2520.
X.-T. Chen, B. Zhou, S. K. Bhattacharya, C. E. Gutteridge, R. R. Pettus Thomas, S. J. Danishefsky, Angew. Chem., Int. Ed., 1998, 37, 789.
X.-T. Chen, C. E. Gutteridge, S. K. Bhattacharya, B. Zhou, R. R. Pettus Thomas, T. Hascall, S. J. Danishefsky, Angew. Chem., Int. Ed., 1998, 37, 185.
K. C. Nicolaou, S. Kim, J. Pfefferkorn, J. Xu, T. Ohshima, S. Hosokawa, D. Vourloumis, T. Li, Angew. Chem., Int. Ed., 1998, 37, 1418.
Y. Lin, C. A. Bewley, J. D. Faulkner, Tetrahedron, 1993, 9, 7977.
T. Shibatani, N. Nishimura, K. Nabe, T. Kakimoto, I. Chibata, Appl. Microbiol., 1974, 27, 225.
E. C. De Fabo, F. P. Noonan, J. Exp. Med., 1983, 158, 84.
F. A. Valeev, O. Yu. Krasnoslobodtseva, L. Kh. Fayzullina, B. T. Sharipov, G. A. Tolstikov, Int. Conf. “Topical Issues of Physical-Organic, Synthetic and Medicinal Chemistry” (21–25 September 2010, Ufa, Russia), 21.
Yu. A. Khalilova, O. Yu. Krasnoslobodtseva, B. T. Sharipov, Yu. S. Galimova, F. A. Valeev, Vestn. Bashkirskogo Gos. Un-ta [Bull. Bashkir Univ.], 2011, 16, 22 (in Russian).
S. E. Sosonyuk, A. Peshich, A. V. Tutushkina, D. A. Khlevin, N. A. Lozinskaya, Yu. A. Gracheva, V. A. Glazunova, D. I. Osolodkin, M. N. Semenova, V. V. Semenov, V. A. Palyulin, M. V. Proskurnina, A. A. Shtil, N. S. Zefirov, Org. Biomol. Chem., 2019, 17, 2792.
N. K. Kashtanova, A. I. Lisina, V. A. Pentegova, Chem. Nat. Compd., 1969, 5, 7.
A. V. Shpatov, M. M. Shakirov, Yu. V. Gatilov, T. V. Rybalova, V. A. Raldugin, Russ. J. Org. Chem., 1997, 33, 194.
F. A. Valeev, Sh. M. Salikhov, O. Yu. Krasnoslobodtseva, B. T. Sharipov, L. V. Spirikhin, G. A. Tolstikov, Chem. Nat. Compd., 2007, 43, 143.
Sh. M. Salikhov, O. Yu. Krasnoslobodtseva, B. T. Sharipov, G. A. Tolstikov, L. V. Spirikhin, F. A. Valeev, Bashkirskii Khim. Zh. [Bashkir Chem. J.], 2007, 14(1), 87 (in Russian).
M. C. Alley, D. A. Scudiero, P. A. Monks, M. L. Hursey, M. J. Czerwinski, D. L. Fine, B. J. Abbott, J. G. Mayo, R. H. Shoemaker, M. R. Boyd, Cancer Res., 1988, 48, 589.
M. R. Grever, S. A. Schepartz, B. A. Chabner, Semin. Oncol., 1992, 19, 622.
M. R. Boyd, K. D. Paull, Drug Dev. Res., 1995, 34, 91.
R. H. Shoemaker, Nature Rev., 2006, 6, 813.
B.-W. Chen, J.-H. Su, C.-F. Dai, P.-J. Sung, Y.-Ch. Wu, Y.-T. Lin, J.-H. Sheu, Bull. Chem. Soc. Jpn, 2011, 84, 1371.
G. Hofle, N. Bedorf, H. Steinmetz, D. Schomburg, K. Gerth, H. Reichenbach, Angew. Chem., 1996, 35, 1567.
D. Meng, P. Bertinato, A. Balog, D.-Sh. Su, T. Kamenecka, E. J. Sorensen, S. J. Danishefsky, J. Am. Chem. Soc., 1997, 119, 10073.
K. C. Nicolaou, S. Ninkovic, F. Sarabia, D. Vourloumis, Y. He, H. Vallberg, M. R. V. Finlay, Z. Yang, J. Am. Chem. Soc., 1997, 119, 7974.
Author information
Authors and Affiliations
Corresponding author
Additional information
The study was financially supported by the Russian Foundation for Basic Research (Project No. 17-43-020166-r_a) and performed within the framework of the state assignment (themes AAAA-A20-120012090028-3 and AAAA-A17-117011910027-0).
Russian Chemical Bulletin, International Edition, Vol. 69, No. 10, pp. 1933–1937, October, 2020
Published in Russian in Izvestiya AkademiiNauk. Seriya Khimicheskaya, No. 10, pp. 1933–1937, October, 2020.
Rights and permissions
About this article
Cite this article
Salikhov, S.M., Faizullina, L.K. & Valeev, F.A. Synthesis and cytotoxic activity of isocembrol and its hydroxy derivatives. Russ Chem Bull 69, 1933–1937 (2020). https://doi.org/10.1007/s11172-020-2981-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11172-020-2981-6