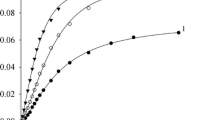
Abstract
The model of selectivity to methyl pelargonate was developed for the hydrocarbomethoxylation of 1-octene catalyzed by the Pd(PPh3)2Cl2—PPh3—p-toluenesulfonic acid system (378 K). The ratio of the rate of methyl pelargonate formation to the sum of the rates of formation of three isomeric esters (reaction products) was accepted as the differential selectivity of the reaction. The model represents a system of equations relating the differential selectivity of the reaction to the CO pressure and concentrations of methanol, PPh3, and p-toluenesulfonic acid. The model adequately depicts the experimental data in a wide range of 1-octene conversions up to 95.5%. The regularities of a change in the reaction selectivity were substantiated using the hydride multiroute mechanism of hydrocarbomethoxylation of 1-octene.
Similar content being viewed by others
References
A. L. Lapidus, S. D. Pirozhkov, Russ. Chem. Rev., 1989, 58, 117.
C. J. Rodriguez, D. F. Foster, G. R. Eastham, D. J. Cole-Hamilton, Chem. Commun., 2004, 1720.
M. Amézquita-Valencia, G. Achonduh, H. Alper, J. Org. Chem., 2015, 80, 6419.
Kh. A. Suerbaev, N. Zh. Kudaibergenov, A. Vavasori, Russ. J. Gen. Chem., 2017, 87, 707.
Kh. A. Suerbaev, N. Zh. Khudaibergenov, A. K. Kurmansitova Russ. J. Gen. Chem., 2016, 86, 2124.
V. A. Aver’yanov, S. A. Batashev, N. T. Sevostianova, V. M. Zarytovsky, Catal. in Ind., 2005, 25.
G. Kiss, Chem. Rev., 2001, 101, 3435.
Yu. G. Noskov, E. S. Petrov, Kinet. Katal., 1994, 35, 728 [Kinet. Catal. (Engl. Transl.), 1994, 35].
E. S. Petrov, Zh. Fiz. Khim., 1988, 62, 2858 [J. Phyz. Chem. USSR (Engl. Transl.), 1988, 62].
I. E. Nifant’ev, S. A. Batashev, S. A. Toloraya, A. N. Tavtorkin, N. T. Sevostyanova, A. A. Vorobiev, V. V. Bagrov, V. A. Averyanov, J. Mol. Catal. A: Chem., 2011, 350, 64.
A. Vavasori, L. Toniolo, G. Cavinato, J. Mol. Catal. A: Chem., 2003, 191, 9.
A. Vavasori, G. Cavinato, L. Toniolo, J. Mol. Catal. A: Chem., 2001, 176, 11.
M. Rosales, I. Pacheco, J. Medira, J. Fernandez, A. Gonzalez, R. Izquierdo, L. G. Melean, P. J. Baricelli, Catal. Lett., 2014, 144, 1717.
S. A. Batashev, Cand. Sci. (Chem.) Dissertation, Tula State University, Tula, 2005, 134 pp. (in Russian).
U. M. Dzhemilev, N. R. Popod’ko, E. V. Kozlova, Metallokompleksnyi kataliz v organicheskom sinteze. Alitsiklicheskie soedineniya [Metallocomplex Catalysis in Organic Synthesis. Alicyclic Compounds], Khimiya, Moscow, 1999, 648 pp. (in Russian).
G. Cavinato, L. Toniolo, A. Vavasori, J. Mol. Catal. A: Chem., 2004, 219, 233.
V. A. Aver’yanov, S. A. Batashev, N. T. Sevost’yanova, N. M. Nosova, Kinet. Catal., 2006, 47, 375.
T. E. Kron, M. I. Terekhova, E. S. Petrov, Kinet. Catal., 2004, 45, 519.
M. Sperrle, G. Consiglio, Chem. Ber./Recl., 1997, 130, 1557.
A. Vavasori, L. Toniolo, J. Mol. Catal. A: Chem., 1996, 110, 13.
R. V. Chaudhari, Industrial Catalytic Processes for Fine and Specially Chemicals, Elsevier Inc., Amsterdam, 2016.
G. Cavinato, L. Toniolo, J. Mol. Catal.: Chem., 1999, 143, 325.
R. Naigre, T. Chenal, I. Ciples, P. Kalck, J. Organomet. Chem., 1994, 480, 91.
M. N. Terekhova, A. B. Sigalov, N. E. Petrova, E. S. Petrov, Zh. Obshch. Khim., 1985, 55, 944 [J. Gen. Chem. USSR (Engl. Transl.), 1985, 55].
V. A. Averyanov, N. T. Sevostyanova, S. A. Batashev, A. A. Vorobiev, A. S. Rodionova, Russ. J. Phys. Chem. B, 2014, 8, 140.
I. E. Nifant’ev, N. T. Sevostyanova, V. A. Averyanov, S. A. Batashev, A. A. Vorobiev, S. A. Toloraya, V. V. Bagrov, A. N. Tavtorkin, Appl. Catal. A: Gen., 2012, 449, 145.
V. A. Aver’yanov, N. T. Sevost’yanova, S. A. Batashev, S. V. Nesol’enaya, Petrol. Chem., 2006, 46, 405.
Yu. G. Noskov, A. I. Simonov, E. S. Petrov, Kinet. Catal., 2000, 41, 511.
Yu. G. Noskov, E. S. Petrov, Kinet. Catal., 1997, 38, 520.
Yu. G. Noskov, E. S. Petrov, Kinet. Katal., 1993, 34, 1005 [Kinet. Catal. (Engl. Transl.), 1993, 34].
Yu. G. Noskov, E. S. Petrov, Russ. Chem. Bull., 2001, 50, 1839.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Russian in Izvestiya Akademii Nauk. Seriya Khimicheskaya, No. 8, pp. 1561–1568, August, 2020.
This work was financially supported by the Government of the Tula Region (Agreement No. DS/130 of October 29, 2018).
Rights and permissions
About this article
Cite this article
Batashev, S.A., Sevostyanova, N.T. Model of selectivity to methyl pelargonate in hydrocarbomethoxylation of 1-octene in the presence of the Pd(PPh3)2Cl2—PPh3—p-toluenesulfonic acid catalytic system. Russ Chem Bull 69, 1561–1568 (2020). https://doi.org/10.1007/s11172-020-2935-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11172-020-2935-z