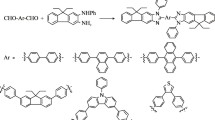
Abstract
The palladium-catalyzed amination was used to synthesize 2,7-diamino derivatives of naphthalene containing two chiral substituents and fluorophore groups (dansyl, 7-methoxycoumarin, 6,7-dimethoxycoumarin, 6-aminoquinoline). The synthesized compounds were studied by UV absorption and fluorescence spectroscopy in the presence of individual enantiomers of amino alcohols and salts of 21 metals. The possibility of using these compounds as fluorescent detectors for optically active compounds and metals was examined. In the presence of (S)-leucinol, the diquinoline derivative showed enhanced emission with a maximum at shorter wavelengths, which is not typical of its (R) isomer. This fact can be used for the recognition of these enantiomers. A number of naphthalene derivatives can be considered as potential fluorescent chemosensors for CuII cations due to the total fluorescence quenching in the presence of this metal.
Similar content being viewed by others
References
F. Puntoriero, G. Bergamini, P. Ceroni, V. Balzani, F. Vogtle, J. Inorg. Organomet. Polym., 2008, 18, 189.
R. Azadbakht, M. Parviz, E. Tamari, H. Keypour, R. Golbedaghi, Spectrochimica Acta, Part A, 2011, 82, 200.
A. M. Costero, M. Colera, P. Gaviña, S. Gil, M. Kubinyi, K. Pál, M. Kállay, Tetrahedron, 2008, 64, 3217.
A. M. Costero, U. Llaosa, S. Gil, M. Parra, M. Colera, Tetrahedron: Asymmetry, 2009, 20, 1468.
A. Pal, P. Besenius, R. P. Sijbesma, J. Am. Chem. Soc., 2011, 133, 12987.
M. I. Burguete, F. Galindo, S. V. Luis, L. Vigara, J. Photochem. Photobiol. A: Chem., 2010, 209, 61.
M. Durmaz, M. Yilmaz, A. Sirit, Org. Biomol. Chem., 2011, 9, 571.
C. Lynam, D. Diamond, J. Mater. Chem., 2005, 15, 307.
X. He, Q. Zhang, W. Wang, L. Lin, X. Liu, X. Feng, Org. Lett., 2011, 13, 804.
P. A. Panchenko, A. S. Polyakova, Yu. V. Fedorov, O. A. Fedorova, Mendeleev Commun., 2019, 29, 155.
S. Pagliari, R. Corradini, G. Galaverna, S. Sforza, A. Dossena, M. Montalti, L. Prodi, N. Zaccheroni, R. Marchelli, Chem. — Eur. J., 2004, 10, 2749.
X. Zhang, J. Yin, J. Yoon, Chem. Rev., 2014, 114, 4918.
O. K. Grigorova, A. D. Averin, O. A. Maloshitskaya, I. P. Beletskaya, Macroheterocycles, 2016, 9, 425.
O. K. Grigorova, A. D. Averin, O. A. Maloshitskaya, I. P. Beletskaya, Macroheterocycles, 2017, 10, 446.
O. K. Grigorova, D. I. Gusev, A. D. Averin, O. A. Maloshitskaya, I. P. Beletskaya, Russ. Chem. Bull., 2019, 68, 848.
A. D. Averin, A. N. Uglov, I. P. Beletskaya, Chem. Lett., 2008, 37, 1074.
J. P. Wolfe, H. Tomori, J. P. Sadighi, J. Yin, S. L. Buchwald, J. Org. Chem., 2000, 65, 1158.
N. M. Chernichenko, V. N. Shevchuk, A. D. Averin, O. A. Maloshitskaya, I. P. Beletskaya, Synlett, 2017, 28, 2800.
T. X. Neenan, G. M. Whitesides, J. Org. Chem., 1988, 53, 2489.
T. Ukai, H. Kawazura, Y. Ishii, J. J. Bonnet, J. A. Ibers, J. Organomet. Chem., 1974, 65, 253.
Author information
Authors and Affiliations
Corresponding author
Additional information
Based on the materials of the XXI Mendeleev Congress on General and Applied Chemistry (September 9–13, 2019, St. Petersburg, Russia).
Published in Russian in Izvestiya Akademii Nauk. Seriya Khimicheskaya, No. 7, pp. 1355–1365, July, 2020.
Rights and permissions
About this article
Cite this article
Malysheva, A.S., Shaferov, A.V., Averin, A.D. et al. Synthesis of optically active 2,7-disubstituted naphthalene derivatives and evaluation of their enantioselective recognition ability. Russ Chem Bull 69, 1355–1365 (2020). https://doi.org/10.1007/s11172-020-2910-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11172-020-2910-8