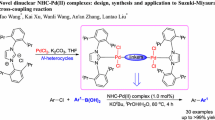
Abstract
Catalytic activity of nickel(ii) and palladium(ii) N-heterocyclic carbene (NHC) complexes derived from imidazole, benzimidazole, and 1,2,4-triazole was comparatively evaluated in the cross-coupling reactions of aryl halides with arylboronic acids. Readily available nickel bis-NHC complexes (NHC)2NiX2 (X = Cl, Br, or I) exhibited the activity comparable to that of the structurally related palladium complexes and, consequently, can be applied as efficient catalysts for the Suzuki—Miyaura reaction.
Similar content being viewed by others
References
N. Miyaura, K. Yamada, A. Suzuki, Tetrahedron Lett., 1979, 20, 3437.
N. Miyaura, A. Suzuki, J. Chem. Soc., Chem. Commun., 1979, 19, 866.
F.-S. Han, Chem. Soc. Rev., 2013, 42, 5270.
I. P. Beletskaya, F. Alonso, V. Tyurin, Coord. Chem. Rev., 2019, 385, 137.
S. E. Hooshmand, B. Heidari, R. Sedghi, R. S. Varma, Green Chem., 2019, 21, 381.
A. Taheri Kal Koshvandi, M. M. Heravi, T. Momeni, Appl. Organomet. Chem., 2018, 32, e4210.
C. Torborg, M. Beller, Adv. Synth. Catal., 2009, 351, 3027.
P. Devendar, R.-Y. Qu, W.-M. Kang, B. He, G.-F. Yang, J. Agric. Food Chem., 2018, 66, 8914.
V. N. Mikhaylov, V. N. Sorokoumov, I. A. Balova, Russ. Chem. Rev., 2017, 86, 459.
A. Yu. Chernenko, A. V. Astakhov, D. V. Pasyukov, P. V. Dorovatovskii, Ya. V. Zubavichus, V. N. Khrustalev, V. M. Chernyshev, Russ. Chem. Bull., 2018, 67, 79.
N. V. Kuchkina, M. Rajadurai, M. Pal, S. Basaveni, S. A. Sorokina, I. Yu. Krasnova, E. S. Serkova, Z. B. Shifrina, Russ. Chem. Bull., 2018, 67, 1035.
S. Z. Tasker, E. A. Standley, T. F. Jamison, Nature, 2014, 509, 299.
V. P. Ananikov, ACS Catal., 2015, 5, 1964.
N. Hazari, P. R. Melvin, M. M. Beromi, Nat. Rev. Chem., 2017, 1, 0025.
Z. N. Gafurov, A. A. Kagilev, A. O. Kantyukov, A. A. Balabaev, O. G. Sinyashin, D. G. Yakhvarov, Russ. Chem. Bull., 2018, 67, 385.
Z. N. Gafurov, A. O. Kantyukov, A. A. Kagilev, A. A. Balabayev, O. G. Sinyashin, D. G. Yakhvarov, Russ. Chem. Bull., 2017, 66, 1529.
D. Balcells, A. Nova, ACS Catal., 2018, 8, 3499.
F. Strieth-Kalthoff, A. R. Longstreet, J. M. Weber, T. F. Jamison, ChemCatChem, 2018, 10, 2873.
Ł. Banach, P. A. Guńka, J. Zachara, W. Buchowicz, Coord. Chem. Rev., 2019, 389, 19.
J. B. Diccianni, T. Diao, Trends Chem., 2019, 1, 830; DOI: https://doi.org/10.1016/j.trechm.2019.08.004.
P. L. Chiu, C.-L. Lai, C.-F. Chang, C.-H. Hu, H. M. Lee, Organometallics, 2005, 24, 6169.
K. Inamoto, J.-i. Kuroda, K. Hiroya, Y. Noda, M. Watanabe, T. Sakamoto, Organometallics, 2006, 25, 3095.
Y. Zhou, Z. Xi, W. Chen, D. Wang, Organometallics, 2008, 27, 5911.
K. Inamoto, J.-i. Kuroda, E. Kwon, K. Hiroya, T. Doi, J. Organomet. Chem., 2009, 694, 389.
M. Nirmala, G. Prakash, R. Ramachandran, P. Viswanathamurthi, J. G. Malecki, W. Linert, J. Mol. Catal. A: Chem., 2015, 397, 56.
S. Wang, F. Ren, Y. Qiu, M. Luo, J. Organomet. Chem., 2015, 788, 27.
S. Gu, J. Du, J. Huang, Y. Guo, L. Yang, W. Xu, W. Chen, Dalton Trans., 2017, 46, 586.
H. V. Huynh, C. Holtgrewe, T. Pape, L. L. Koh, E. Hahn, Organometallics, 2006, 25, 245.
J. Berding, J. A. van Paridon, V. H. S. van Rixel, E. Bouwman, Eur. J. Inorg. Chem., 2011, 2450.
A. V. Astakhov, O. V. Khazipov, E. S. Degtyareva, V. N. Khrustalev, V. M. Chernyshev, V. P. Ananikov, Organometallics, 2015, 34, 5759.
K. Zhang, M. Conda-Sheridan, S. R. Cooke, J. Louie, Organometallics, 2011, 30, 2546.
J. C. Bernhammer, H. V. Huynh, Organometallics, 2014, 33, 5845.
K. Matsubara, S. Miyazaki, Y. Koga, Y. Nibu, T. Hashimura, T. Matsumoto, Organometallics, 2008, 27, 6020.
H. Valdés, M. Poyatos, G. Ujaque, E. Peris, Chem. Eur. J., 2015, 21, 1578.
C. D. Abernethy, A. H. Cowley, R. A. Jones, J. Organomet. Chem., 2000, 596, 3.
C. J. O’Brien, E. A. B. Kantchev, C. Valente, N. Hadei, G. A. Chass, A. Lough, A. C. Hopkinson, M. G. Organ, Chem. Eur. J., 2006, 12, 4743.
A. V. Astakhov, O. V. Khazipov, A. Y. Chernenko, D. V. Pasyukov, A. S. Kashin, E. G. Gordeev, V. N. Khrustalev, V. M. Chernyshev, V. P. Ananikov, Organometallics, 2017, 36, 1981.
S. Gupta, B. Basu, S. Das, Tetrahedron, 2013, 69, 122.
V. M. Chernyshev, A. V. Astakhov, I. E. Chikunov, R. V. Tyurin, D. B. Eremin, G. S. Ranny, V. N. Khrustalev, V. P. Ananikov, ACS Catal., 2019, 9, 2984.
D. B. Eremin, V. P. Ananikov, Coord. Chem. Rev., 2017, 346, 2.
E. G. Gordeev, D. B. Eremin, V. M. Chernyshev, V. P. Ananikov, Organometallics, 2018, 37, 787.
O. V. Khazipov, M. A. Shevchenko, A. Y. Chernenko, A. V. Astakhov, D. V. Pasyukov, D. B. Eremin, Y. V. Zubavichus, V. N. Khrustalev, V. M. Chernyshev, V. P. Ananikov, Organometallics, 2018, 37, 1483.
E. A. Denisova, D. B. Eremin, E. G. Gordeev, A. M. Tsedilin, V. P. Ananikov, Inorg. Chem., 2019, 58, 12218.
D. Eremin, E. Denisova, A. Kostyukovich, J. Martens, G. Berden, J. Oomens, V. Khrustalev, V. Chernyshev, V. P. Ananikov, Chem. Eur. J., 2019, 25, 16564.
V. M. Chernyshev, O. V. Khazipov, M. A. Shevchenko, A. Yu. Chernenko, A. V. Astakhov, D. B. Eremin, D. V. Pasyukov, A. S. Kashin, V. P. Ananikov, Chem. Sci., 2018, 9, 5564.
G. A. Abakumov, A. V. Piskunov, V. K. Cherkasov, I. L. Fedushkin, V. P. Ananikov, D. B. Eremin, E. G. Gordeev, I. P. Beletskaya, A. D. Averin, M. N. Bochkarev, A. A. Trifonov, U. M. Dzhemilev, V. A. D’yakonov, M. P. Egorov, A. N. Vereshchagin, M. A. Syroeshkin, V. V. Jouikov, A. M. Muzafarov, A. A. Anisimov, A. V. Arzumanyan, Yu. N. Kononevich, M. N. Temnikov, O. G. Sinyashin, Yu. H. Budnikova, A. R. Burilov, A. A. Karasik, V. F. Mironov, P. A. Storozhenko, G. I. Shcherbakova, B. A. Trofimov, S. V. Amosova, N. K. Gusarova, V. A. Potapov, V. B. Shur, V. V. Burlakov, V. S. Bogdanov, M. V. Andreev, Russ. Chem. Rev., 2018, 87, 393.
A. Yu. Chernenko, D. V. Pasyukov, A. V. Astakhov, V. A. Tafeenko, V. M. Chernyshev, Russ. Chem. Bull., 2018, 67, 1196.
K. Cavell, Dalton Trans., 2008, 47, 6676.
A. V. Astakhov, S. B. Soliev, E. G. Gordeev, V. M. Chernyshev, V. P. Ananikov, Dalton Trans., 2019, 48, 17052.
T. Tu, H. Mao, C. Herbert, M. Xu, K. H. Dotz, Chem. Commun., 2010, 46, 7796.
Ł. Banach, P. A. Guńka, D. Gõrska, M. Podlewska, J. Zachara, W. Buchowicz, Eur. J. Inorg. Chem., 2015, 5677.
O. R. Luca, B. A. Thompson, M. K. Takase, R. H. Crabtree, J. Organomet. Chem., 2013, 730, 79.
K. Matsubara, K. Ueno, Y. Shibata, Organometallics, 2006, 25, 3422.
Author information
Authors and Affiliations
Corresponding author
Additional information
The authors are grateful to Academician of the Russian Academy of Sciences V. P. Ananikov for the fruitful discussion of the results and valuable comments. The authors are also grateful to the Shared Research Center “Nanotech nologies” of the M. I. Platov South-Russian State Polytechnic University (NPI) and the Department of Structural Studies of the N. D. Zelinsky Institute of Organic Chemistry of the Russian Academy of Sciences for carrying out the analytical experiments.
This work was financially supported by the Russian Foundation for Basic Research (Project No. 16-29-10786).
Based on the materials of the International Conference “Catalysis and Organic Synthesis” (ICCOS-2019) (September 15–20, 2019, Moscow, Russia).
Published in Russian in Izvestiya Akademii Nauk. Seriya Khimicheskaya, No. 4, pp. 0683–0690, April, 2020.
Rights and permissions
About this article
Cite this article
Soliev, S.B., Astakhov, A.V., Pasyukov, D.V. et al. Nickel(ii) N-heterocyclic carbene complexes as efficient catalysts for the Suzuki—Miyaura reaction. Russ Chem Bull 69, 683–690 (2020). https://doi.org/10.1007/s11172-020-2818-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11172-020-2818-3