Abstract
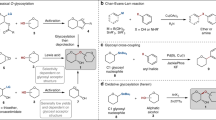
Efficient multigram two-step syntheses of 4-(2-chloroethoxy)phenol and 4-(3-chloropropoxy)phenol in >70% yields starting from 4-hydroxybenzaldehyde and reagents with general formula Cl(CH2) n X (X = Cl, n = 2; X = OTs, n = 3) are proposed. 4-(2-Chloroethoxy)phenol can also be conveniently prepared from 4-methoxyphenol and 1,2-dichloroethane. The compounds thus obtained can be used in carbohydrate chemistry to synthesize glycosides bearing "universal" 4-(ω-chloroalkoxy)phenyl aglycons.
Similar content being viewed by others
References
H. K. A. C. Coolen, H. Engelkamp, J. N. H. Reek, A. H. Priem, P. W. N. M. van Leeuwen, R. J. M. Nolte, Recl. Trav. Chim. Pays-Bas, 1995, 114, 65.
B. Batanero, R. Saez, F. Barba, Electrochim. Acta, 2009, 54, 4872.
R. Mondal, C. Guha, A. K. Mallik, Tetrahedron Lett., 2014, 55, 86.
E. Blume, E. Winkelmann, W. Schaper, W. Raether, W. Dittmar, 1-(2-Aryl-1,3-dioxolan-2-ylmethyl)-azole, ihre Salze, Verfahren zu ihrer Herstellung, sie enthaltende Zubereitungen und ihre Verwendung, Pat. DE 3410070; Chem. Abstr., 1985, 104, 68865g.
P. I. Abronina, K. G. Fedina, N. M. Podvalnyy, A. I. Zinin, A. O. Chizhov, N. N. Kondakov, V. I. Torgov, L. O. Kononov, Carbohydr. Res., 2014, 396, 25.
N. M. Podvalnyy, P. I. Abronina, E. L. Zdorovenko, A. O. Chizhov, A. I. Zinin, V. I. Torgov, L. O. Kononov, Russ. Chem. Bull. (Int. Ed.), 2014, 63, 497 [Izv. Akad. Nauk, Ser. Khim., 2014, 497].
N. N. Kondakov, T. M. Melnikova, A. I. Zinin, V. I. Torgov, A. O. Chizhov, E. A. Gordeeva, N. V. Bovin, L. O. Kononov, Russ. Chem. Bull. (Int. Ed.), 2014, 63, 501 [Izv. Akad. Nauk, Ser. Khim., 2014, 501].
N. N. Kondakov, T. M. Melnikova, T. V. Chekryzhova, M. V. Melnikova, A. I. Zinin, V. I. Torgov, A. O. Chizhov, L. O. Kononov, Russ. Chem. Bull. (Int. Ed.), 2015, 64, 1142 [Izv. Akad. Nauk, Ser. Khim., 2015, 1142].
P. I. Abronina, A. I. Zinin, D. A. Romashin, N. N. Malysheva, A. O. Chizhov, L. O. Kononov, Synlett, 2015, 26, 2267.
N. M. Podvalnyy, A. O. Chizhov, A. I. Zinin, L. O. Kononov, Carbohydr. Res., 2016, 431, 25.
Z. Zhang, G. Magnusson, Carbohydr. Res., 1996, 295, 41.
A. Ya. Chernyak, G. V. M. Sharma, L. O. Kononov, P. Radha Krishna, A. B. Levinsky, N. K. Kochetkov, A. V. Rama Rao, Carbohydr. Res., 1992, 223, 303.
E. Cavero, M. Zablocka, A.-M. Caminade, J. P. Majoral, Eur. J. Org. Chem., 2010, 2759.
G. Magnusson, A. Ya. Chernyak, J. Kihlberg, L. O. Kononov, in Neoglycoconjugates: Preparation and Application, Eds Y. C. Lee, R. T. Lee, Acad. Press, San Diego, 1994, p. 53.
T. K. Lindhorst, Beilstein J. Org. Chem., 2014, 10, 2345–2347 (and references cited therein).
Multivalent glycosystems for nanoscience, Beilstein J. Org. Chem., Ed. T. K. Lindhorst, 2014, 10, 2345–2376.
D. Solís, N. V. Bovin, A. P. Davis, J. Jiménez-Barbero, A. Romero, R. Roy, K. Smetana, Jr., H.-J. Gabius, Biochim. Biophys. Acta, Gen. Subj., 2015, 1850, 186.
M. Matsumoto, K. Kobayashi, Y. Hotta, J. Org. Chem., 1984, 49, 4740.
L. Katz, L. S. Karger, W. Schroeder, M. S. Cohen, J. Org. Chem., 1953, 18, 1380.
M. R. Patel, A. Bhatt, J. D. Steffen, A. Chergui, J. Murai, Y. Pommier, J. M. Pascal, L. D. Trombetta, F. R. Fronczek, T. T. Talele, J. Med. Chem., 2014, 57, 5579.
J. White, C. G. Whiteley, Synthesis, 1993, 1141.
A. Ouach, S. Gmouh, M. Pucheault, M. Vaultier, Tetrahedron, 2008, 64, 1962.
M. Schmidt, J. Ungvári, J. Glöde, B. Dobner, A. Langner, Bioorg. Med. Chem., 2007, 15, 2283.
A. N. Kursunlu, E. Guler, H. I. Ucan, R. W. Boyle, Dyes Pigm., 2012, 94, 496.
G. M. O. Amombo, T. Kramer, F. L. Monte, S. Göring, M. Fach, S. Smith, S. Kolb, R. Schubenel, K. Baumann, B. Schmidt, Bioorg. Med. Chem. Lett., 2012, 22, 7634.
S. Manohar, S. I. Khan, S. K. Kandi, K. Raj, G. Sun, X. Yang, A. D. C. Molina, N. Ni, B. Wang, D. S. Rawat, Bioorg. Med. Chem. Lett., 2013, 23, 112.
E. Reinholz, A. Becker, B. Hagenbruch, S. Schäfer, A. Schmitt, Synthesis, 1990, 1069.
G. J. Kemperman, T. A. Roeters, P. W. Hilberink, Eur. J. Org. Chem., 2003, 1681.
K.-S. Yeung, M. E. Farkas, Z. Qiu, Z. Yang, Tetrahedron Lett., 2002, 43, 5793.
G. R. Harris, R. Stewart, Can. J. Res., Sect. B, 1949, 27, 739.
D. Enders, G. Geibel, S. Osborne, Chem. Eur. J., 2000, 6, 1302.
J. J. Li, C. Limberakis, D. A. Pflum, Modern Organic Synthesis in the Laboratory, Oxford Univ. Press, New York, 2007, p. 19.
R. J. Alabaster, I. F. Cottrell, H. Marley, S. H. B. Wright, Synthesis, 1988, 950.
M. Le Naour, V. Leclerc, A. Farce, D.-H. Caignard, N. Hennuyer, B. Staels, V. Audinot-Bouchez, J.-A. Boutin, M. Lonchampt, C. Dacquet, A. Ktorza, P. Berthelot, N. Lebegue, ChemMedChem, 2012, 7, 2179.
S. Corsano, G. Strappaghetti, A. Codagnone, R. Scapicchi, G. Marucci, Eur. J. Med. Chem., 1992, 27, 545.
T. Taguri, R. Yamakawa, Y. Adachi, K. Mori, T. Ando, Biosci., Biotechnol., Biochem., 2010, 74, 119.
S. C. Suri, J. C. Marcischak, Org. Prep. Proced. Int., 2013, 45, 154.
W. H. Edgerton, R. L. Hull, J. R. Fisher, J. Am. Pharm. Assoc., 1959, 48, 320.
A. V. Gromyko, K. V. Popov, A. P. Mosoleva, S. A. Streltsov, S. L. Grokhovsky, V. A. Oleinikov, A. L. Zhuze, Russ. J. Bioorg. Chem. (Engl. Transl.), 2005, 31, 344 [Bioorg. Khim., 2005, 31, 385].
W. Jiang, K. Nowosinski, N. L. Löw, E. V. Dzyuba, F. Klautzsch, A. Schäfer, J. Huuskonen, K. Rissanen, C. A. Schalley, J. Am. Chem. Soc., 2012, 134, 1860.
M. Kueny-Stotz, R. Brouillard, S. Chassaing, Synlett, 2012, 23, 2053.
B. Willhalm, A. F. Thomas, F. Gautschi, Tetrahedron, 1964, 20, 1185.
S. E. Yagunov, S. V. Khol´shin, N. V. Kandalintseva, A. E. Prosenko, Russ. Chem. Bull. (Int. Ed.), 2013, 62, 1395 [Izv. Akad. Nauk, Ser. Khim., 2013, 1395].
T. R. Bell-Horwath, A. K. Vadukoot, F. S. Thowfeik, G. Li, M. Wunderlich, J. C. Mulloy, E. J. Merino, Bioorg. Med. Chem. Lett., 2013, 23, 2951.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Russian in Izvestiya Akademii Nauk. Seriya Khimicheskaya, No. 2, pp. 0304—0312, February, 2017.
Rights and permissions
About this article
Cite this article
Zinin, A.I., Stepanova, E.V., Jost, U. et al. An efficient multigram-scale synthesis of 4-(ω-chloroalkoxy)phenols. Russ Chem Bull 66, 304–312 (2017). https://doi.org/10.1007/s11172-017-1732-9
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11172-017-1732-9