Abstract
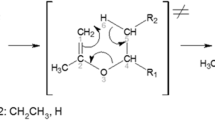
The quantum chemical modeling and topological analysis of transition states of the concerted molecular decomposition of haloalkanes and alcohols with the elimination of HHal and HOH were performed for the ten compounds. The possibility of formation of two types of transition states was mentioned, and their electronic structures were studied. The influence of the alkyl substituent at the C atoms on the binding energy and the nature of the transition state was shown. The decomposition activation energies were calculated using the method of density functional theory (DFT/B3LYP/6-311++G**) and the intersecting parabolas method. The calculated results were compared, and their agreement was shown.
Similar content being viewed by others
References
C. K. Ingold, Structure and Mechanism in Organic Chemistry, Cornell University Press, London, 1969, 1266 pp.
E. T. Denisov, O. M. Sarkisov, G. I. Likhtenshtein, Chemical Kinetcs: Fundamentals and New Developments, Elsevier, Amsterdam, 2003, p. 268.
N. N. Semenov, Izbrannye trudy. T. 3. O nekotorykh problemakh khimicheskoi kinetiki i reaktsionnoi sposobnosti [Selected Works. Vol. 3. On Some Problems of Chemical Kinetics and Reactivity], Nauka, Moscow, 2005, p. 37 (in Russian).
G. B. Sergeev, V. V. Smirnov, Molekulyarnoe galogenirovanie olefinov [Molecular Halogenation of Olefins], Izd-vo MGU, Moscow, 1985, 240 pp. (in Russian).
S. W. Benson, Thermochemical Kinetics: Methods for the Estimation of Thermochemical Data and Rate Parameters, Wiley and Sons, New York, 1968.
E. S. Swinbourne, in Comprehensive Chemical Kinetics, Vol. 5, Eds C. H. Bamford, C. F. H. Tipper, Elsevier, Amsterdam, 1972, p. 149.
S. W. Benson, H. E. O’Neal, Kinetic Data on Gas Phase Unimolecular Reactions, NSRDS–NBS 21, Gaithersburg, 1970, p. 628.
T. S. Pokidova, E. T. Denisov, Russ. J. Phys. Chem. B (Engl. Transl.), 2016, 10, 394 [Khim. Fiz., 2016, 35, 23].
T. S. Pokidova, Tez. dokl. IX Vseros. konf. “Vysokoreaktsionnye intermediaty khimicheskikh i biokhimicheskikh reaktsii (ChemInt–2014)” [Proc. IX All-Russia Conf. “Highly Reactive Intermediates of Chemical and Biochemical Reactions (ChemInt–2014)”] Moscow Region, October 20–23, 2014), Moscow, 2014, p. 48 (in Russian).
T. S. Pokidova, E. T. Denisov, A. F. Shestakov, Petroleum Chem. (Engl. Transl.), 2009, 49, 363 [Neftekhimiya, 2009, 49, 343].
E. T. Denisov, T. S. Pokidova, Russ. Chem. Rev., 2012, 81, 415.
T. S. Pokidova, E. T. Denisov, Kinet. Catal. (Engl. Transl.), 2001, 42, 729 [Kinet. Katal., 2001, 42, 805].
L. L. Bladow, C. J. Stopera, W. D. Thweatt, M. Page, J. Phys. Chem. A., 2010, 114, 4304.
M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery, J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, T. Keith, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, O. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski, D. J. Fox, Gaussian 09, Revision D.01, Gaussian, Inc., Wallingford (CT), 2013.
T. A. Keith, AIMAll (Version 15.05.18), TK Gristmill Software, Overland Park KS, USA, 2015 (aim.tkgristmill.com).
E. T. Denisov, General Aspects of the Chemistry of Radicals, Ed. Z. B. Alfassi, Wiley, Chichester, England,1999, p. 79.
E. T. Denisov, T. G. Denisova, T. S. Pokidova, Handbook of Free Radical Initiators, Wiley–Interscience, Hoboken, New York, 2003.
I. V. Aleksandrov, Teoret. Eksp. Khim. [Theor. Exp. Chem.], 1976, 12, 878 (in Russian).
NIST Chemistry WebBook, NIST Standard Reference Database Number 69; http://webbook.nist.gov/chemistry/
NIST Standard Reference Database 19A, Positive Ion Energetics, Version 2.02, Gaithersburg, 1994.
S. W. Benson, Thermochemical Kinetics, Wiley, New York, 1976, 320 pp.
E. S. Domalski, E. D. Hearing, J. Phys. Chem. Ref. Data, 1993, 22, 805.
R. F. W. Bader, Atoms in Molecules. A Quantum Theory, Clarendon Press, Oxford, 1990.
M. A. Turpin, K. C. Smith, G. L. Heard, D. W. Setser, B. E. Holmes, J. Phys. Chem. A, 2014, 118, 9347.
C. L. Parworth, M. K. Tucker, B. E. Holmes, G. L. Heard, J. Phys. Chem. A, 2011, 115, 13133.
E. Espinosa, E. Molins, C. Lecomte, Chem. Phys. Lett., 1998, 285, 170.
V. I. Minkin, B. Ya. Simkin, R. M. Minyaev, Teoriya stroeniya molekul [Theory of Molecular Structure], Feniks, Rostov-onDon,1997, p. 89 (in Russian).
W. H. Richardson, H. E. O’Neal, in Comprehensive Chemical Kinetics, Eds C. H. Bamford, C. F. H. Tipper, Elsevier, Amsterdam, 1972, 5, 381.
Author information
Authors and Affiliations
Corresponding author
Additional information
Dedicated to the 60th anniversary of the Institute of Problems of Chemical Physics, Russian Academy of Sciences.
Published in Russian in Izvestiya Akademii Nauk. Seriya Khimicheskaya, No. 10, pp. 2333—2339, October, 2016.
Rights and permissions
About this article
Cite this article
Emel´yanova, N.S., Pokidova, T.S. Topological analysis of transition states of the concerted molecular decomposition of haloalkanes and alcohols with HHal and HOH elimination. Russ Chem Bull 65, 2333–2339 (2016). https://doi.org/10.1007/s11172-016-1585-7
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11172-016-1585-7