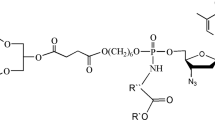
Abstract
A series of phosphoramidate derivatives of β-L-2´,3´-dideoxy-3´-thiacytidine (lamivudine, 3TC), which are the latent forms of 3TC monophosphate, were synthesized. An optimal synthetic pathway towards these compounds involves synthesis of phosphoramidates from diphenyl phosphite followed by treating the resulting 3TC phenyl phosphite with tetrachloromethane and different amines. Surprisingly, this reaction sequence produces two groups of products, 3TC phenyl phosphoramidates (4) and bis-3TC-phosphoramidates (5). Synthesis of compounds 5 has not been previously described.
Similar content being viewed by others
References
J. H. Brehm, D. L. Koontz, C. L. Wallis, K. A. Shutt, I. Sanne, R. Wood, J. A. McIntyre, W. S. Stevens, N. SluisCremer, J. W. Mellors, Clin. Infect. Dis., 2012, 55, 737–745.
B. Groschel, N. Himmel, J. Cinatl, C. Perigaud, G. Gosselin, J. L. Imbach, H. W. Doerr, J. Cinatl, Jr., Nucleosides Nucleotides, 1999, 18, 921–926
J. Balzarini, O. Wedgwood, J. Kruining, H. Pelemans, R. Heijtink, E. De Clercq, C. McGuigan, Biochem. Biophys. Res. Commun., 1996, 225, 363–369.
N. F. Zakirova, A. V. Shipitsyn, M. V. Jasko, M. M. Prokofjeva, V. L. Andronova, G. A. Galegov, V. S. Prassolov, S. N. Kochetkov, Bioorg. Med. Chem., 2012, 20, 5802–5809.
N. F. Zakirova, I. L. Karpenko, M. M. Prokofjeva, K. Vanpouille, V. S. Prassolov, A. V. Shipitsyn, S. N. Kochetkov, Russ. Chem. Bull. (Int. Ed.), 2014, 63, 1192–1196 [Izv. Akad. Nauk, Ser. Khim., 2014, 1192–1196].
A. V. Shipitsyn, N. F. Zakirova, E. F. Belanov, T. R. Pronyaeva, N. V. Fedyuk, M. K. Kukhanova, A. G. Pokrovsky, Nucleosides, Nucleotides Nucleic Acids, 2003, 22, 963–966.
N. F. Zakirova, A. V. Shipitsyn, M. V. Jasko, S. N. Kochetkov, Russ. J. Bioorg. Chem. (Engl. Transl.), 2011, 37, 578–585 [Bioorg. Khim., 2011, 37, 645-653].
C. McGuigan, K. Madela, M. AlJarah, C. Bourdin, M. Arrica, E. Barrett, S. Jones, A. Kolykhalov, B. Bleiman, K. D. Bryant, B. Ganguly, E. Gorovits, G. Henson, D. Hunley, J. Hutchins, J. Muhammad, A. Obikhod, J. Patti, C. R. Walters, J. Wang, J. Vernachio, C. V. S. Ramamurty, S. K. Battina, S. Chamberlain, J. Med. Chem., 2011, 54, 8632–8645.
F. R. Atherton, H. T. Openshaw, A. R. Todd, J. Chem. Soc., 1945, 660–663.
M. Neisius, S. Liang, H. Mispreuve, S. Gaan, Ind. Eng. Chem. Res., 2013, 52, 9752–9762.
E. M. Georgiev, J. Kaneti, K. Troev, D. M. Roundhill, J. Am. Chem. Soc., 1993, 115, 10964–10973.
S. S. Le Corre, M. Berchel, H. Couthon-Gourves, J.-P. Haelters, P.-A. Jaffres, Beilstein J. Org. Chem., 2014, 10, 1166–1196.
S. Cao, Y. Zhao, Sci. Sin. Chim., 2015, 45, 283–294.
V. Mitova, N. Koseva, K. Troev, RSC Adv., 2014, 4, 64733–64736.
A. Kers, I. Kers, J. Stawifiski, M. Sobkowski, A. Kraszewski, Tetrahedron, 1996, 52, 9931–9944.
Author information
Authors and Affiliations
Corresponding author
Additional information
Dedicated to Academician of the Russian Academy of Sciences N. S. Zefirov on the occasion of his 80th birthday.
Published in Russian in Izvestiya Akademii Nauk. Seriya Khimicheskaya, No. 9, pp. 2167–2171, September, 2015.
Rights and permissions
About this article
Cite this article
Zakirova, N.F., Shipitsyn, A.V. Lamivudine phosphoramidates. Unexpected products of a well-known reaction. Russ Chem Bull 64, 2167–2171 (2015). https://doi.org/10.1007/s11172-015-1133-x
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11172-015-1133-x