Abstract
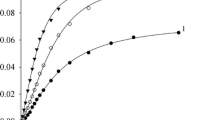
The quantitative regularities of the effect of CO pressure and temperature on the rate of cyclohexene hydrocarbomethoxylation catalyzed by the Pd(OAc)2-PPh3-TsOH system were defined. Extremal dependences of the reaction rate on the CO pressure were revealed in the temperature range from 353 to 383 K. To interpret the obtained results, the catalytic cycle was constructed which included the hydride, alkyl, and acyl palladium complexes of the cationic type as intermediates. It was proposed that the catalyst is partially converted into an inactive form due to the exchange between ligands. The experiments on the effect of the CO pressure on the reaction rate made it possible to estimate the apparent rate constants for the kinetic reaction equation obtained earlier.
Similar content being viewed by others
References
G. Kiss, Chem. Rev., 2001, 101, 3435.
E. Drent, P. H. M. Buzelaar, Chem. Rev., 1996, 96, 663.
E. S. Petrov, Yu. G. Noskov, Ross. Khim. Zh., 1998, 42,No. 4, 149 [Mendeleev Chem. J. (Engl. Transl.), 1998, 42, No. 4].
G. Cavinato, L. Toniolo, J. Mol. Catal. A: Chem., 1979, 6, 111.
E. Nagy, B. Heil, S. Törös, J. Mol. Catal. A: Chem., 1999, 143, 229.
E. G. Chepaikin, A. P. Bezruchenko, A. A. Leshcheva, Kinet. Catal. (Engl. Transl.), 2006, 40, 313 [Kinet. Katal., 1999, 40, 348].
A. Vavasori, G. Cavinato, L. Toniolo, J. Mol. Catal. A: Chem., 2001, 176, 11.
A. Vavasori, L. Toniolo, G. Cavinato, J. Mol. Catal. A: Chem., 2003, 191, 9.
N. T. Sevost’yanova, S. A. Batashev, V. A. Aver’yanov, A. M. Demerlii, Petroleum Chem. (Engl. Transl.), 2012, 52, 35 [Neftekhimiya, 2012, 52, 39].
V. A. Aver’yanov, N. T. Sevost’yanova, S. A. Batashev, A. M. Demerlii, Petroleum Chem. (Engl. Transl.), 2013, 53, 39 [Neftekhimiya, 2013, 53, 43].
V. A. Aver’yanov, N. T. Sevost’yanova, S. A. Batashev, Petroleum Chem. (Engl. Transl.), 2008, 48, 287 [Neftekhimiya, 2008, 48, 286].
A. R. Elman, V. A. Matveev, E. V. Slivinskii, S. M. Loktev, Pharm. Chem. J. (Engl. Transl.), 1990, 24,No. 3, 217 [Khim.-Farm. Zh., 1990, 24, No. 3, 47].
I. E. Nifantév, S. A. Batashev, S. A. Toloraya, A. N. Tavtorkin, N. T. Sevostyanova, A. A. Vorobiev, V. V. Bagrov, V. A. Averyanov, J. Mol. Catal. A: Chem., 2011, 350, 64.
V. A. Aver’yanov, S. A. Batashev, N. T. Sevost’yanova, N. M. Nosova, Kinet. Catal. (Engl. Transl.), 2006, 47, 375 [Kinet. Katal., 2006, 47, 381].
V. A. Aver’yanov, N. T. Sevost’yanova, S. A. Batashev, S. V. Nesolenaya, Petroleum Chem. (Engl. Transl.), 2006, 46, 405 [Neftekhimiya, 2006, 46, 435].
N. T. Sevost’yanova, A. A. Vorob’ev, V. A. Aver’yanov, S. A. Batashev, Tez. dokl. XIV Mezhdunar. nauchno-tekhn. konf. “Naukoemkie khimicheskie tekhnologii-2012” [Proc. XIV Intern. Scientific Technical Conf. “Chemical High Technologies”] (Tula, May 21–25, 2012), Izd-vo MITKhT, Moscow, 2012, 92 (in Russian).
V. A. Aver’yanov, N. T. Sevost’yanova, S. A. Batashev, Izv. Vuz. Khim. Khim. Tekhnol. [Bulletin of Higher Educational Institutions. Chemistry and Chemical Technology], 2008, 55,No. 4, 111 (in Russian).
A. S. Rodionova, V. A. Averyanov, N. T. Sevostyanova, A. A. Vorobiev, S. A. Batashev, Intern. Symp. “Modern Trends in Organometallic Chemistry and Catalysis” dedicated to the 90th anniversary of the Acad. M. E. Volpin (Moscow, June 3–7, 2013), Abstrs, Moscow, 2013, 147.
I. E. Nifantév, N. T. Sevostyanova, V. A. Averyanov, S. A. Batashev, A. A. Vorobiev, S. A. Toloraya, V. V. Bagrov, A. N. Tavtorkin, Appl. Catal. A: General, 2012, 449, 145.
M. I. Terekhova, A. B. Sigalov, N. E. Petrova, E. S. Petrov, J. Gen. Chem. USSR (Engl. Transl.), 1985, 55, 944 [Zh. Obshch. Khim., 1985, 55, 944].
G. Verspui, I. I. Moiseev, R. A. Sheldon, J. Organomet. Chem., 1999, 586, 196.
R. Bardi, A. M. Piazzasi, G. Cavinato, L. Toniolo, Inorg. Chim. Acta, 1985, 102, 99.
M. I. Terekhova, N. E. Petrova, R. R. Shifrina, E. S. Petrov, J. Gen. Chem. USSR (Engl. Transl.) 1988, 58, 581 [Zh. Obshch. Khim., 1988, 58, 658].
G. Cavinato, L. Toniolo, A. Vavasori, J. Mol. Catal. A: Chem., 2004, 219, 233.
V. A. Aver’yanov, N. T. Sevost’yanova, S. A. Batashev, A. A. Vorob’ev, A. S. Rodionova, Russ. J. Phys. Chem. B (Engl. Transl.), 2014, 8,No. 2, 140 [Khim. Fiz., 2014, 33, No. 3, 19].
Author information
Authors and Affiliations
Corresponding author
Additional information
According to the materials of the International Symposium “Modern Trends in Organometallic Chemistry and Catalysis“ (June 3–7, 2013, Moscow).
Published in Russian in Izvestiya Akademii Nauk. Seriya Khimicheskaya, No. 4, pp. 0837–0842, April, 2014.
Rights and permissions
About this article
Cite this article
Sevostyanova, N.T., Averyanov, V.A., Batashev, S.A. et al. Effect of temperature and CO pressure on the rate of cyclohexene hydrocarbomethoxylation catalyzed by the Pd(OAc)2-PPh3-TsOH system. Russ Chem Bull 63, 837–842 (2014). https://doi.org/10.1007/s11172-014-0518-6
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11172-014-0518-6