Abstract
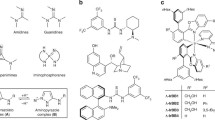
Schiff bases derived from (1R,2R)-1,2-diaminocyclohexane and 1 eq. of salycylic (or substituted salycylic) aldehyde form stereochemically inert positively charged chiral octahedral Coiii complexes of Δ-configuration with the stereoselectivity approaching 100%. To evaluate the calatylic activity and stereoinduction of the resulting complexes with various counteranions in the outer sphere, a model reaction of trimethylsilyl cyanide addition to benzaldehyde was used. O-trimethylsilylmandelonitrile formed in the process had an enantiomeric purity up to 27%. Complexes with F− counterion showed high catalytic activity.
Similar content being viewed by others
References
H. J. Federsel, K. M. J. Brands, Org. Process Res. Dev., 2007, 11, 494.
H. Hugl, Asymmetric Synthesis: The Essentials, 2nd ed., Eds M. Christmann, S. Bräse, Wiley-VCH Verlag GmbH & Co. KgaA, Weinheim, 2008, p. 307.
H. Yamamoto, C. H. Cheon, Chiral Lewis Acids and Brønsted Acids in Asymmetric Synthesis, in Catalytic Asymmetric Synthesis, Ed. I. Ojima, John Wiley & Sons, Inc. 2010, p. 119.
P. M. Pihko, H. Rahaman, in Enantioselective Organocatalyzed Reactions I, Ed. R. Mahrwald, Springer, Heidelberg, 2011, 185–207.
J. G. Hernandez, E. Juaristi, Chem. Commun., 2012, 48, 5396.
S. E. Denmark, N. D. Gould, L. M. Wolf, J. Org. Chem., 2011, 76, 4337.
T. Marcelli, J. H. van Maarseveen, H. Hiemstra, Angew. Chem., Int. Ed. (Engl.), 2006, 45, 7496.
A. G. Doyl, E. N. Jacobsen, Chem. Rev., 2007, 107, 5713.
T. Akiyama, Chem. Rev., 2007, 107, 5744.
M. Terada, Synthesis, 2010, 1929, as well as articles cited therein.
U. Koch, P. L. A. Popelier, J. Phys. Chem., 1995, 99, 9747.
G. A. Jeffrey, An introduction to Hydrogen Bonding, Oxford University Press, 1997.
E. Espinosa, E. Molins, C. Lecomte, Chem. Phys. Lett., 1998, 285, 170.
J.-A. van den Berg, K. R. Seddon, Cryst. Growth Des., 2003, 3, 643–661.
H. Miyabe, Y. Takemoto, Bull. Chem. Soc. Jpn, 2008, 81, 785.
H. Yamamoto, K. Futatsugi, Angew. Chem., Int. Ed. Engl., 2005, 44, 1924; C. H. Choen, H. Yamamoto, Chem. Commun., 2011, 47, 3043.
Y. N. Belokon, B. Green, N. S. Ikonnikov, V. S. Larichev, B. V. Lokshin, M. A. Moscalenko, M. North, C. Orizu, A. S. Peregudov, G. I. Timofeeva, Eur. J. Org. Chem., 2000, 2655.
F. G. Bordwell, Acc. Chem. Res., 1988, 21, 456.
R. Goumont, E. Magnier, E. Kizilian, F. Terrier, J. Org. Chem., 2003, 68, 6566, as well as articles cited therein.
A. D. Dilman, V. V. Levin, M. Karni, Y. Apeloig, J. Org. Chem., 2006, 71, 7214, as well as articles cited therein.
H. F. Bauer, W. C. Drinkard, J. Am. Chem. Soc., 1960, 82, 5031.
H.-J. Schanz, M. A. Linseis, D. G. Gilheany, Tetrahedron Asymmetry, 2003, 14, 2763.
G. M. Sheldrick, SADABS, v. 2.03, Bruker/Siemens Area Detector Absorption Correction Program; Bruker AXS Inc., Madison, WI, 2003.
G. M. Sheldrick, Acta Crystallogr. Sect. A, 2008, 64, 112.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Russian in Izvestiya Akademii Nauk. Seriya Khimicheskaya, No. 12, pp. 2231–2239, December, 2012.
Rights and permissions
About this article
Cite this article
Belokon, Y.N., Larionov, V.A., Mkrtchyan, A.F. et al. A novel type of catalysts for the asymmetric C-C bond formation based on chiral stereochemically inert cationic Coiii complexes. Russ Chem Bull 61, 2252–2260 (2012). https://doi.org/10.1007/s11172-012-0320-2
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11172-012-0320-2