Abstrac
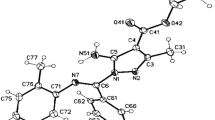
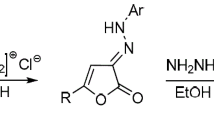
The methods for selective synthesis of regioisomeric 4-amino- and 6-amino-1-aryl-3-R-1H-pyrazolo[3,4-b]pyridine-5-carbonitriles were developed. Heating 4-amino-substituted pyrazolo[3,4-b]pyridines gave 6-amino-substituted isomers in quantitative yield. Spectral differences characteristic of both isomers were evaluated based on the 13C NMR spectra.
Similar content being viewed by others
References
J. Elguero, Comprehensive Heterocyclic Chemistry, Eds A. R. Katritzky, C. W. Rees, E. F. V. Scriven, Pergamon, New York, 1996.
J. Elguero, P. Goya, N. Jagerovic, A. M. S. Silva, Targets Heterocycl. Syst., 2002, 6, 52.
V. A. Chebanov, K. A. Gura, S. M. Desenko, Top. Heterocycl. Chem., 2010, 23, 41.
J. Shi, G. Xu, W. Zhu, H. Ye, S. Yang, Y. Luo, J. Han, J. Yang, R. Li, Y. Wei, L. Chen, Bioorg. Med. Chem. Lett., 2010, 20, 4273.
N. R. Mohamed, N. Y. Khaireldin, A. F. Fahmy, A. A. El-Sayed, Pharma Chemica, 2010, 2, 400.
M. S. M. Carla, M. R. S. A. Carlos, R. R. Carlos, J. B. Eliezer, J. Mol. Struct. (Theochem.), 2002, 579, 31.
B. Leal, I. F. Afonso, C. R. Rodrigues, P. A. Abreu, R. Garrett, L. C. S. Pinheiro, A. R. Azevedo, J. C. Borges, P. F. Vegi, C. C. C. Santos, F. C. A. da Silveira, L. M. Cabral, I. C. P. P. Frugulhetti, A. M. R. Bernardino, D. O. Santos, H. C. Castro, Bioorg. Med. Chem., 2008, 16, 8196.
F. A. Goda, A. A.-M. Abdel-Aziz, O. A. Attef, Bioorg. Med. Chem., 2004, 12, 1845.
R. B. Geraldo, M. L. Bello, L. R. S. Dias, M. A. F. Vera, T. Nagashima, P. A. Abreu, M. B. Santos, M. G. Albuquerque, L. M. Cabral, A. C. C. Freitas, M. V. Kalil, C. R. Rodrigues, H. C. Castro, J. Atheroscler. Thromb., 2010, 17, 730.
D.-S. Su, J. J. Lim, E. Tinney, B.-L. Wan, M. B. Young, K. D. Anderson, D. Rudd, V. Munshi, C. Bahnck, P. J. Felock, M. Lu, M.-T. Lai, S. Touch, G. Moyer, D. J. DiStefano, J. A. Flynn, Y. Yuexia Liang, R. Sanchez, R. Perlow-Poehnelt, M. M. Miller, J. P. Vacca, T. M. Williams, N. J. Anthony, J. Med. Chem., 2009, 52, 7163.
G. A. Nishiguchi, G. Atallah, C. Bellamacina, M. T. Burger, Y. Ding, P. H. Feucht, P. D. Garcia, W. Han, L. Klivansky, M. Lindvall, Bioorg. Med. Chem. Lett., 2011, 21, 6366.
S. T. Staben, T. P. Heffron, D. P. Sutherlin, S. R. Bhat, G. M. Castanedo, I. S. Chuckowree, J. Dotson, A. J. Folkes, L. S. Friedman, L. L. Lee, J. Lesnick, C. Lewis, J. M. Murray, J. Nonomiya, A. G. Olivero, E. Plise, J. Pang, W. W. Wei Prior, L. L. Salphati, L. L. Rouge, D. D. Sampath, V. V. Tsui, N. C. N. C. Wan, S. S. Wang, C. C. Weismann, P. Wu, B.-Y. Zhu, Bioorg. Med. Chem. Lett., 2010, 20, 6048.
E. J. Barreiro, C. A. Camara, H. Verli, L. Brazil-Más, N. G. Castro, W. M. Cintra, Y. Aracava, C. R. Rodrigues, C. A. M. Fraga, J. Med. Chem., 2003, 46, 1144.
F. Shi, Q. Wang, S. Tu, J. Zhou, B. Jiang, C. Li, D. Zhou, Q. Shao, L. Cao, J. Heterocycl. Chem., 2008, 45, 1103.
C.-L. Shi, D.-Q. Shi, S. H. Kim, Z.-B. Huang, S.-J. Ji, M. Ji, Tetrahedron, 2008, 64, 2425.
A. Shaabani, M. Seyyedhamzeh, A. Maleki, M. Behnam, F. Rezazadeh, Tetrahedron Lett., 2009, 50, 17.
J. Quiroga, J. Portilla, H. Serrano, R. Abonia, B. Insuasty, M. Nogueras, J. Cobo, Tetrahedron Lett., 2007, 48, 1987.
Z. Airong, Z. Wei, P. Jinhui, Synth. Commun., 2006, 36, 1549.
M. N. Jachak, A. B. Avhale, V. J. Medhane, R. B. Toche, J. Heterocycl. Chem., 2006, 43, 1169.
R. B. Toche, D. C. Bhavsar, M. A. Kazi, S. M. Bagul, M. N. Jachak, J. Heterocycl. Chem., 2010, 47, 287.
N. A. Hamdy, A. M. Gamal-Eldeen, Eur. J. Med. Chem., 2009, 44, 4547.
G. Lavecchia, S. Berteina-Raboin, G. Guillaumet, Tetrahedron Lett., 2004, 45, 6633; G. Lavecchia, S. Berteina-Raboin, G. Guillaumet, Tetrahedron Lett., 2004, 45, 2389.
S. G. Cottis, P. B. Clarke, H. Tieckelmann, J. Heterocycl. Chem., 1965, 2, 192.
L. Revesz, E. Blum, F. E. Di Padova, T. Buhl, R. Feifel, H. Gram, P. Hiestand, U. Manning, U. Neumann, G. Rucklin, Bioorg. Med. Chem. Lett., 2006, 16, 262; G. L. Beutner, J. K. Kuethe, M. M. Kim, N. Yasuda, J. Org. Chem., 2009, 74, 789.
G. P. Sagitullina, L. A. Lisitskaya, M. A. Vorontsova, S. Reva, R. S. Sagitullin, Mendeleev Commun., 2007, 17, 192.
S. Lee, S. B. Park, Org. Lett., 2009, 11, 5214.
H. Stankovičová, A. Gáplovsky, M. Lácová, J. Chovancová, A. Puchała, J. Heterocycl. Chem., 2006, 43, 843.
E. E. Emelina, A. A. Petrov, S. I. Selivanov, D. V. Filyukov, Zh. Org. Khim., 2008, 44, 259 [Russ. J. Org. Chem. (Engl. Transl.), 2008, 44, 251].
A. M. Hussein, T. I. El-Emary, J. Chem. Res. S, 1998, 20.
B. M. Lynch, M. A. Khan, N. C. Teo, F. Pedrotti, Can. J. Chem., 1988, 66, 420.
S. M. Al-Mousawi, K. Kaul, M. A. Mohammad, M. H. Elnagdi, J. Chem. Res. M, 1997, 2026.
T. I. El-Emary, J. Chin. Chem. Soc. (Taipei), 2007, 46, 585.
V. K. Ahluwalia, B. Goyal, Synth. Commun., 1996, 26, 1341.
A. Bondi, J. Phys. Chem., 1964, 68, 441.
I. I. Granberg, N. F. Krokhina, Khim.-Farm. Zh., 1968, 16 [Pharm. Chem. J. (Engl. Transl.), 1968, 12].
N. L. Nam, I. I. Granberg, V. I. Sorokin, Khim. Geterotsikl. Soedin., 2000, 342 [Chem. Heterocycl. Compd. (Engl. Transl.), 2000, 36, 281]; C. Alberti, C. Tironi, Farmaco, 1964, 19, 618; J. Portilla, E. G. Mata, M. Nogueras, J. Cobo, J. N. Low, C. Glidewell, Acta Crystallogr., Sect. C, 2007, 63, O21.
Bruker AXS, APEX2 — Software Suite for Crystallographic Programs, Bruker AXS, Madison (USA), 2009.
G. M. Sheldrick, Acta Crystallogr., 2008, A64, 112.
L. J. Farrugia, J. Appl. Crystallogr., 1999, 32, 837.
G. M. Sheldrick, SADABS — Bruker AXS Scaling and Absorption Correction, Bruker AXS, Madison (USA), 2008.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Russian in Izvestiya Akademii Nauk. Seriya Khimicheskaya, No. 4, pp. 0886-0991, April, 2012.
Rights and permissions
About this article
Cite this article
Petrov, A.A., Kasatochkin, A.N., Emelina, E.E. et al. Regioisomeric 4-amino- and 6-aminopyrazolo[3,4-b]pyridines: synthesis and structure determination by NMR spectroscopy and X-ray diffraction. Russ Chem Bull 61, 891–896 (2012). https://doi.org/10.1007/s11172-012-0125-3
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11172-012-0125-3