Abstract
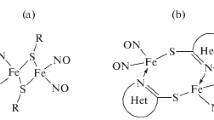
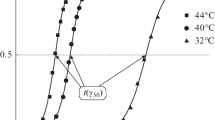
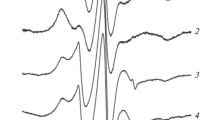
The kinetics of erythrocyte hemolysis and intra-erythrocyte hemoglobin oxidation under the action of synthetic sulfur-nitrosyl iron complexes was studied. The complexes capable of releasing nitric oxide due to spontaneous hydrolytic decomposition was studied. The addition of these complexes to a 0.2% suspension of mouse erythrocytes results in hemolysis. The kinetic curves of hemolysis exhibit an induction period, whose duration is different for each complex. The hemolysis is preceded by hemoglobin oxidation with nitric oxide penetrating into the cell. The oxidation of hemoglobin follows the first-order rate equation. The apparent first-order rate constants characterizing the NO-donating ability of each complex were determined. The hemolytic effect of the studied complexes is suggested to be related to the formation of peroxynitrite inside erythrocytes. Peroxynitrite is the cytotoxic product of interaction of nitric oxide and the superoxide radical anion.
Similar content being viewed by others
References
N. S. Bryan, K. Bian, F. Murad, Frontiers in Bioscience, 2009, 14, 1.
P. Pacher, J. S. Beckman, L. Liaudet, Physiol. Rev., 2007, 87, 315.
D. A. Wink, K. M. Miranda, M. G. Espey, Exp. Biol. Med. (Maywood, NJ, USA), 2001, 226, 621.
N. A. Sanina, S. M. Aldoshin, Izv. Akad. Nauk, Ser. Khim., 2004, 2326 [Russ. Chem. Bull., Int. Ed., 2004, 53, 2428].
N. A. Sanina, L. A. Syrtsova, N. I. Shkondina, T. N. Rudneva, E. S Malkova, T. A. Bazanov, A. I. Kotel’nikov, S. M. Aldoshin, Nitric Oxide, 2007, 16, 181.
J. D. Young, L. G. Leong, M. A. DiNome, Z. A. Gohn, Anal. Biochem., 1986, 154, 649.
A. Ilani, R. Granoth, Biochim. Biophys. Acta, Biomembr., 1990, 1027, 199.
W. G. Zijlstra, A. Buursma, W. P. Meeuwsen-van der Roest, Clin. Chem. (Washington, DC, USA), 1991, 37, 1633.
R. F. Eich, T. Li, D. D. Lemon, D. H. Doherty, S. R. Curry, J. F. Aitken, A. J. Mathews, K. A. Jonson, R. D. Smith, G. N. J. Phillips, J. S Olson, Biochemistry, 1996, 35, 6976.
X. Liu, M. J. S. Miller, M. S. Joshi, H. Sadowska-Krowicka, D. A. Clark, J. R. Lancaster, J. Biol. Chem., 1998, 273, 18709.
J. S. Beckman, T. W. Beckman, J. Chen, P. A. Marshall, B. A. Freeman, Proc. Natl. Acad. Sci. USA, 1990, 87, 1620.
H. Kondo, M. Takahashi, E. Niki, FEBS Lett., 1997, 413, 236.
G. Ferrer-Sueta, R. Radi, ACS Chem. Biol., 2009, 4, 161.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Russian in Izvestiya Akademii Nauk. Seriya Khimicheskaya, No. 12, pp. 2160–2163, December, 2010.
Rights and permissions
About this article
Cite this article
Neshev, N.I., Psikha, B.L., Sokolova, E.M. et al. Kinetic regularities of erythrocyte hemolysis and hemoglobin oxidation under the action of sulfur-nitrosyl iron complexes as nitric oxide donors. Russ Chem Bull 59, 2215–2218 (2010). https://doi.org/10.1007/s11172-010-0381-z
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11172-010-0381-z